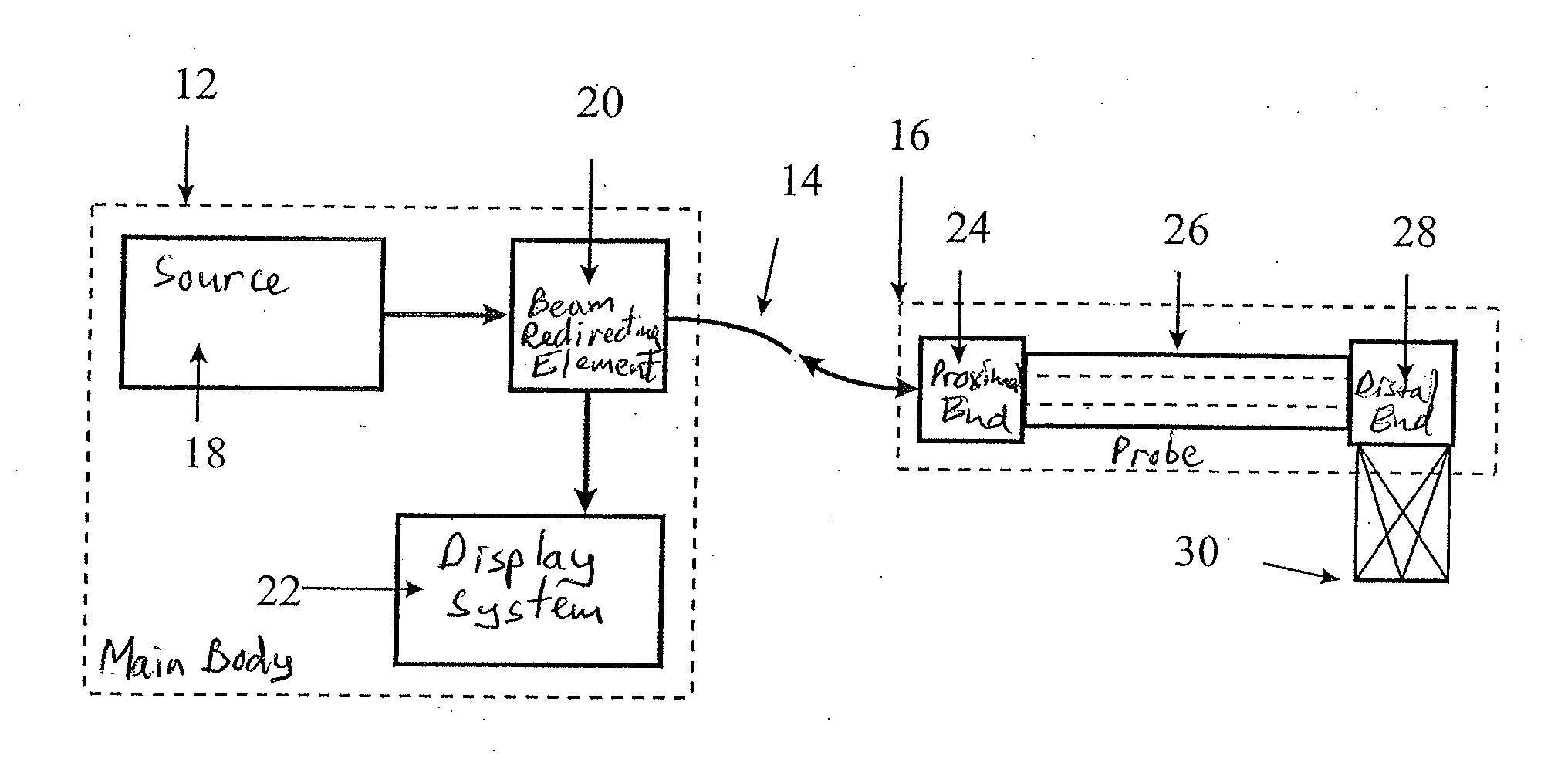
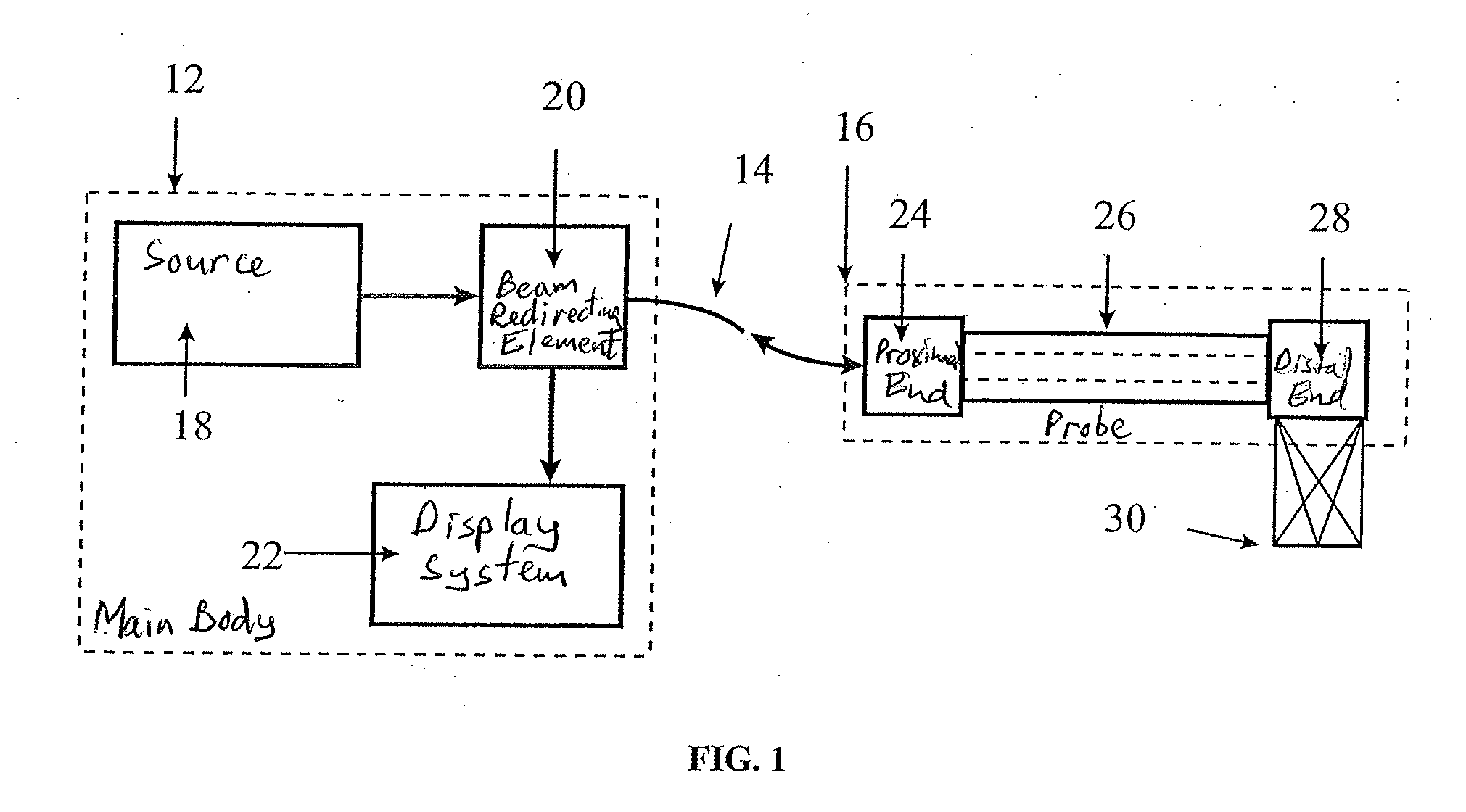
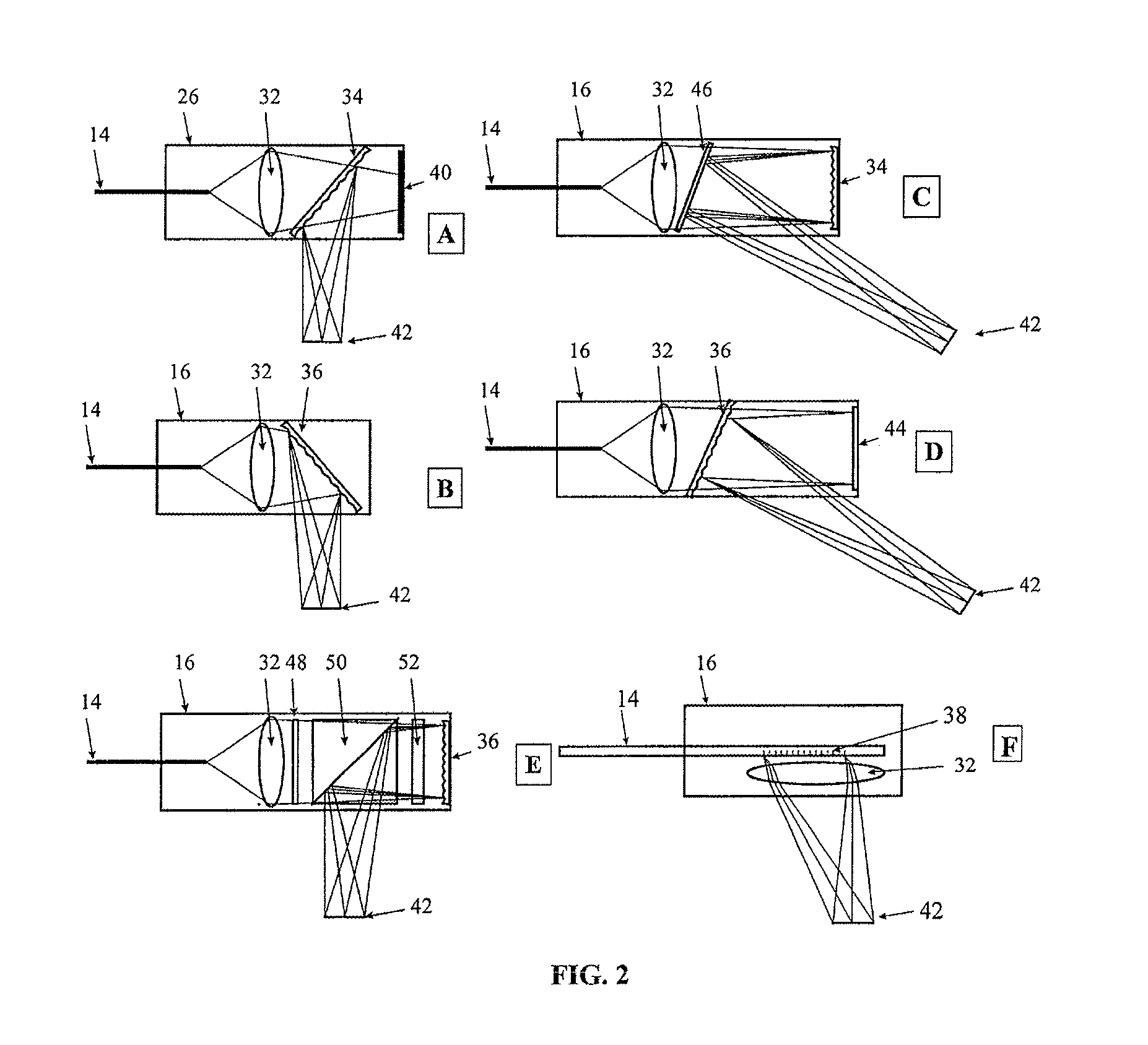
Spectrally encoded miniature endoscopic imaging probe
a technology of endoscope and imaging probe, which is applied in the field of endoscopy, can solve the problems of limitations, disadvantages and limitations of using conventionally available endoscopes, and currently available probes limiting their use to certain procedures and locations,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
color embodiment
[0058]In an alternative embodiment of the present invention, shown in FIG. 5, a device 100 can be constructed that uses at least two and preferably three or more separate broadband source modules 110, for example, three sources centered at red (630 nm) 102, green (540 nm) 104, and blue (480 nm) 106 to produce color images using this technique. It is to be understood that other colors, wavelengths and number of separate sources can be selected depending on various factors, including, but not limited to, the imaging environment, imaging target, measurements to be obtained, and the like. The three energy components can be separated after reflection from the sample 30 and recombined to form an image. Each of the source / detector modules 102, 104 and 106 for the three spectral bands transmits selected wavelength light to an optical mixer / separator 108, which selectively transmits the light toward the imaging head 109 and to the imaging optics 110 for the different colors. The light reflec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com