Binder for lithium ion rechargeable battery cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Electrodes and Testing of Binders
[0039]A series of binders were tested by making up anodes using silicon powder as the active material, the binder set out in Table 2 and a conductive carbon black (Super P® carbon black obtained from TIMCAL, Strada Industriale, CH-6743 Bodio, Switzerland, or Denka Black obtained from Denka (Denki Kagaku Kogyo Kabushiki Kaisha, Tokyo) or a mixture thereof) in a ratio of silicon active material:binder:carbon black of 80:8:12 (wt %) or 76:12:12 (wt %). The polymer solutions are pre-made by dissolving the polymer solid material into the appropriate solvent either water or an organic solvent, as set out in Table 2. Specific composite mixes commence with dispersion of the relevant wt. % of the Si active material into a 10-15 wt % bead-milled solution of the carbon black (Super P carbon or Denka Black) by shear stirring for 12 hours. The relevant wt % of polymer solution is then added to this and the resulting composite is subjected to Dual A...
example 2
Measuring First Cycle Loss
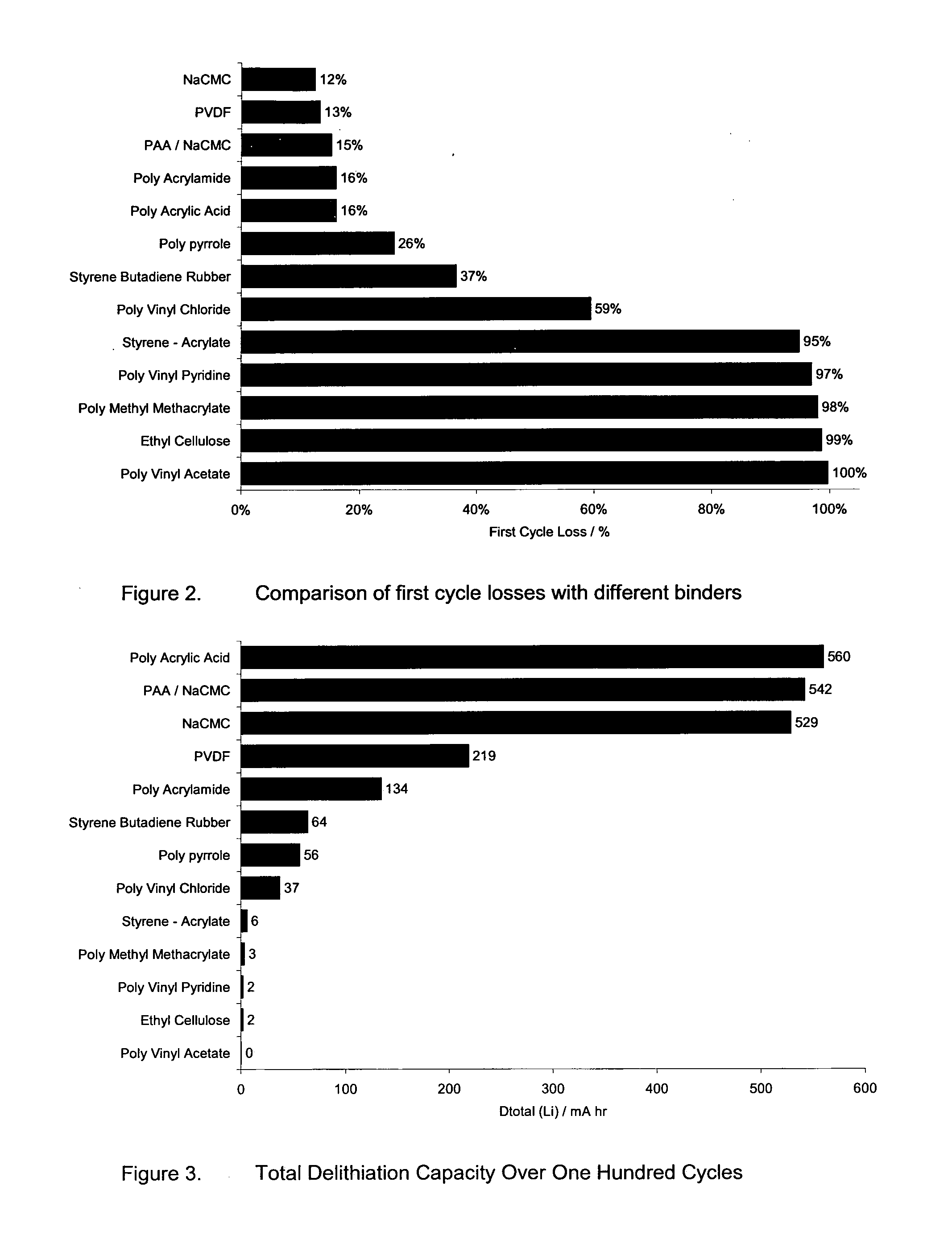
[0050]Using the same cell structure and method of manufacture as in Example 1, cells with various binders as per Table 2 were formed and tested for FCL. The results of the FCL tests for the various binders are shown in the bar chart of FIG. 2. It should be noted that Table 2 includes a wider range of experiments including the different composition ratios such as 74:13:13 whereas FIG. 2 is based on a standard formulation of 80:8:12.
example 3
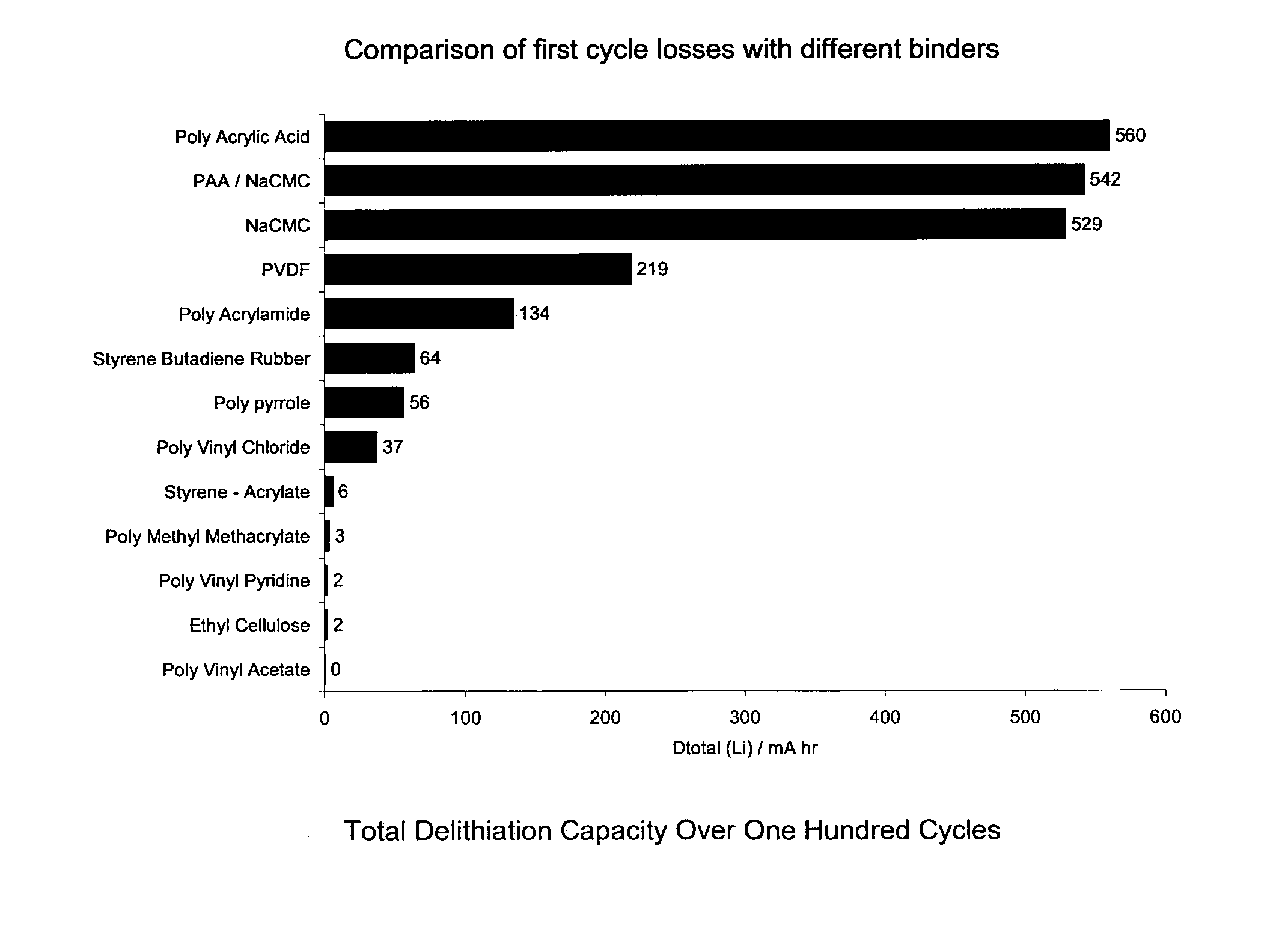
[0051]Using the same cell structure and method of manufacture as in Example 1, cells with various binders were formed as per Table 2 and tested to find the effect of the anode binder on the cycling capacity and the results are shown in the bar chart of FIG. 3. FIG. 3 shows the total delithiation capacity for silicon powder composite electrodes with a lithium metal counter electrode. Delithiation capacity is the amount of lithium capacity in mA hr from the test sample cells associated with the electrochemical step equivalent to the discharge in a real Li-ion cell (i.e. where lithium is removed from the silicon material) The total delithiation capacity is the cumulative amount of capacity from the all the cycles up to the point where the test cell was deemed to have failed.
[0052]Lithium metal electrodes have a limited cycle life, because of the porous and non-uniform deposits that form when lithium is plated back on to the anode during recharging. Typically, the total amount of capaci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com