Liquid organic semiconductor material
a semiconductor material and liquid organic technology, applied in semiconductor devices, solid-state devices, thermoelectric devices, etc., can solve the problems that organic materials in liquid state cannot achieve organic electronic devices, the conductivity of such substances is experimentally confirmed, etc., to reduce the production cost of devices, increase the application range of devices, and increase the effect of surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
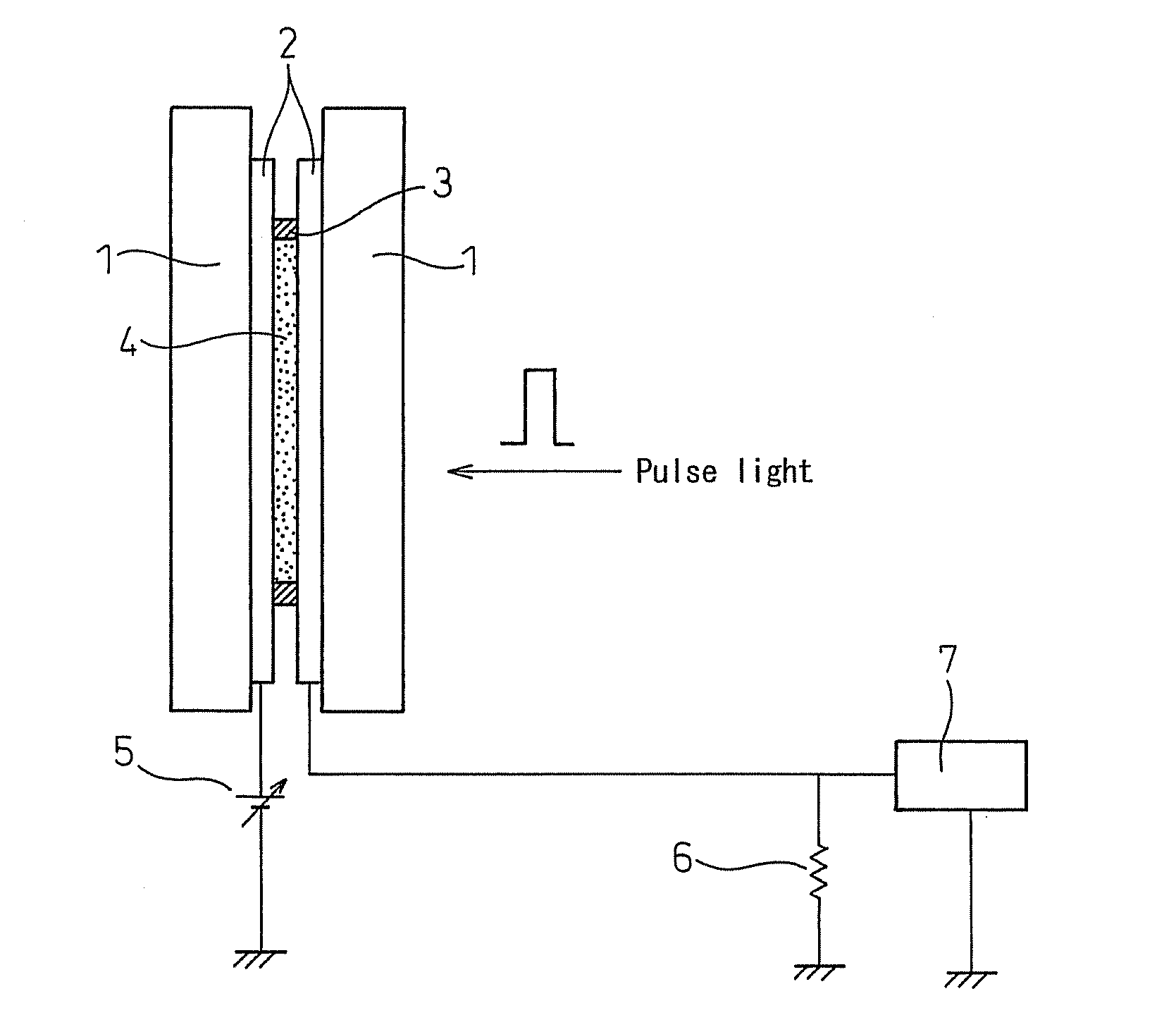
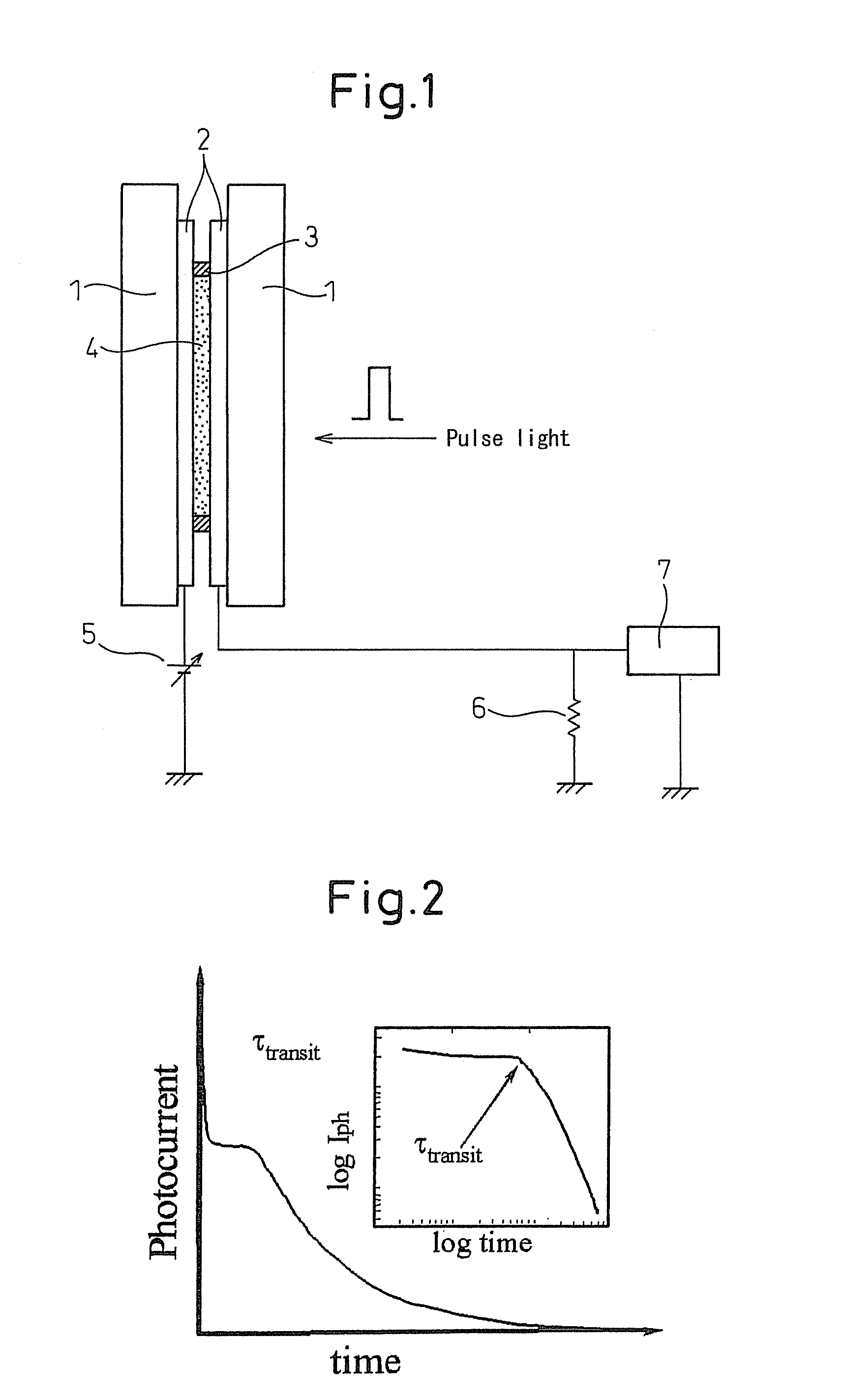
[0087](The mobility of the material is 10−3 cm2 / Vs or more, and thus the material does not need to be considered as one that causes the ionic conduction.)
[0088]A purified TPD (N,N′-diphenyl-N,N′-bis(3-methylphenyl)-[1,1′-diphenyl]-4,4′-diamine) was injected into a liquid crystal cell at the temperature of the isotropic phase. The transient photocurrent was measured by the above time-of-flight method to determine a mobility of the material from the transit time of the charge. The mobilities of a positive charge and a negative charge obtained at a measurement temperature of 150° C. were 4×10−3 cm2 / Vs and 4×10−3 cm2 / Vs, respectively. From the mobilities, the conduction can be determined to be not the ionic conduction, but the electronic conduction caused by each of the hole and the electron.
[0089]In the same manner, in the purified TTA(tritolylamine), the mobilities of the positive charge and the negative charge at 100° C. were determined to be 4×10−3 cm2 / Vs and 9×10−5 cm2 / Vs, respecti...
example 2
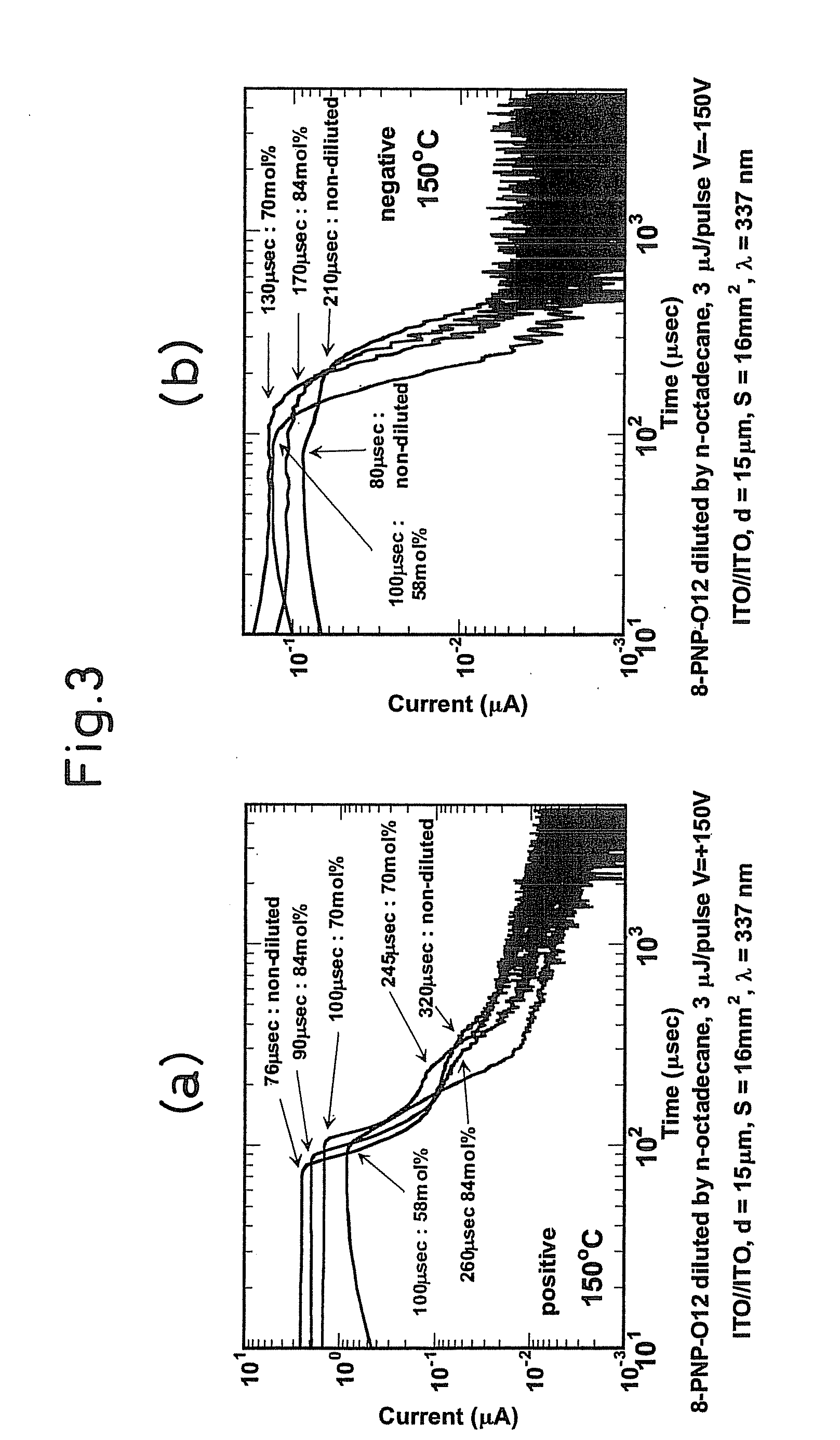
[0091]A 6-(4′-octylphenyl)-2-dodecyloxynaphtalene(8-PNP-012) was injected in an isotropic phase (liquid phase: in a thickness of the sample of 15 μm.) into a glass cell having an ITO electrode (4 mm square) positioned therein. A 337 nm nitrogen laser pulsed light (pulse width: 600 ps, 3 μJ / pulse) was radiated to apply voltages of +150 V or −150 V to the electrode on the light irradiation side, so that the transient photocurrent observed was measured by a digital oscilloscope. The wave profiles (in black) shown in FIG. 5 (whose left side illustrated the waveform of a transient photocurrent of the positive charge, and whose right side illustrated the waveform of a transient photocurrent of the negative charge) had two shoulders corresponding to transit times in different time regions. In order to clarify the conduction, the dilution experiment using an n-octadecane was performed. When the concentration of the n-octadecane was changed from 16 mol % to 42 mol %, the change of waveform o...
example 3
[0094]A 2-phenylnaphthalene in an isotropic phase (liquid phase: in a thickness of the sample of 16.31 μm) was charged into a glass cell having an ITO electrode (4 mm square) positioned therein in the same manner as in Example 2. A 337 nm nitrogen laser pulsed light (pulse width: 600 ps, 3 μJ / pulse) was radiated at 105° C. to apply voltages of +10 to 100 V or −10 to 100 V to the electrode on the light irradiation side, so that the transient photocurrent was observed and measured by a digital oscilloscope. The waveform of the transient photocurrent was shown in FIG. 5. The waveform of the positive charge has one stepped part representing a charge travelling in an early time region. The waveform of the negative charge has “two shoulders” corresponding to two different travelling time regions.
[0095]In the same manner as in Example 2, According to the result of the dilution experiment, the mobilities of the hole and electron were determined to be 8.9×10−4 cm2 / Vs, and 8.8×10−4 cm2 / Vs, re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com