Compositions and methods of generating reprogrammed adipocyte cells and methods of use therefore
a technology of adipocyte cells and adipocytes, which is applied in the direction of optical radiation measurement, chemical vapor deposition coating, fungi, etc., can solve the problems of adverse health effects and mortality, and insufficient methods for preventing or treating obesity and associated metabolic disorders, so as to reduce the probability of developing a disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reprogrammed Adipocyte Cells were Obtained by the Directed Differentiation of Human Embryonic Stem Cells
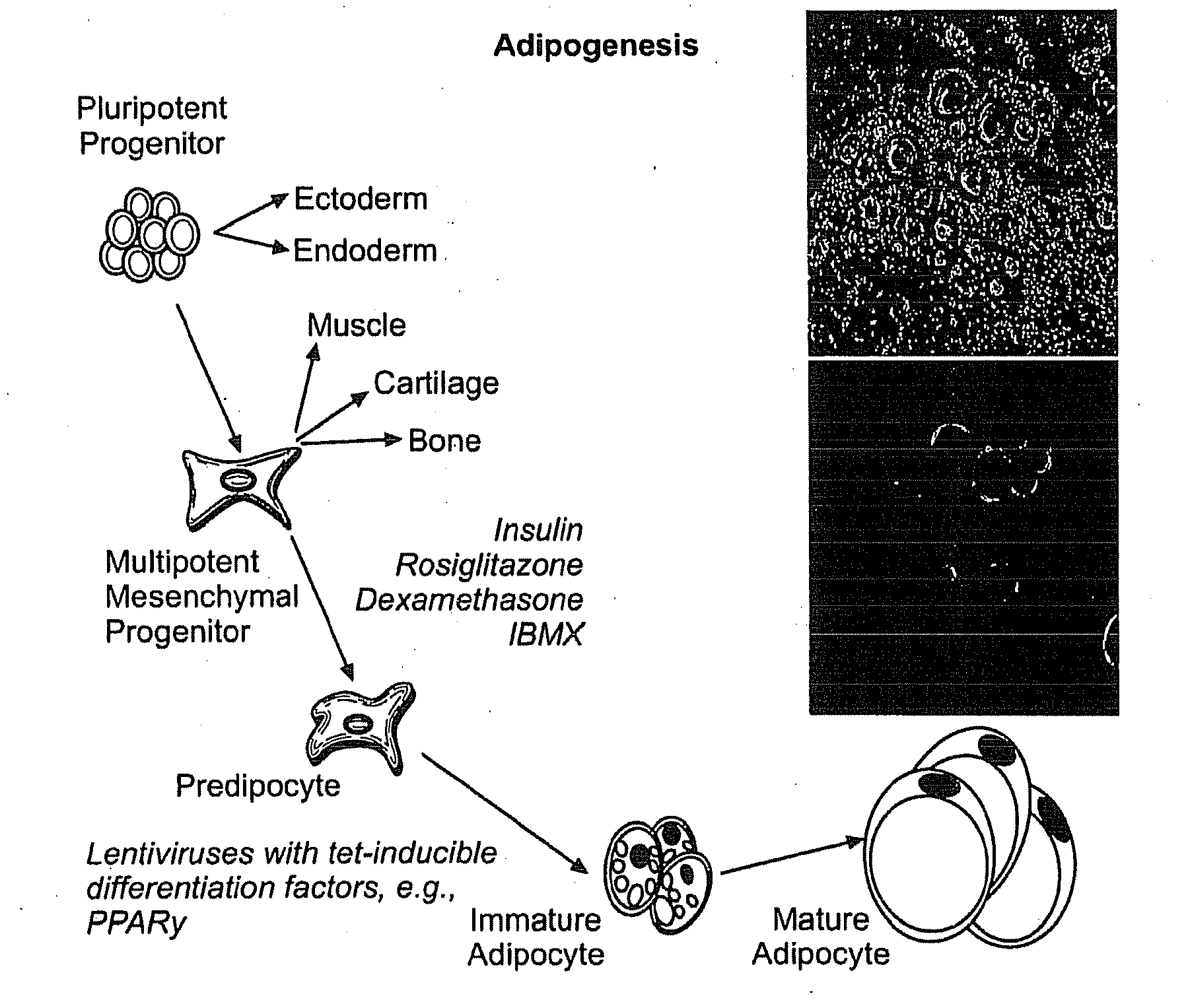
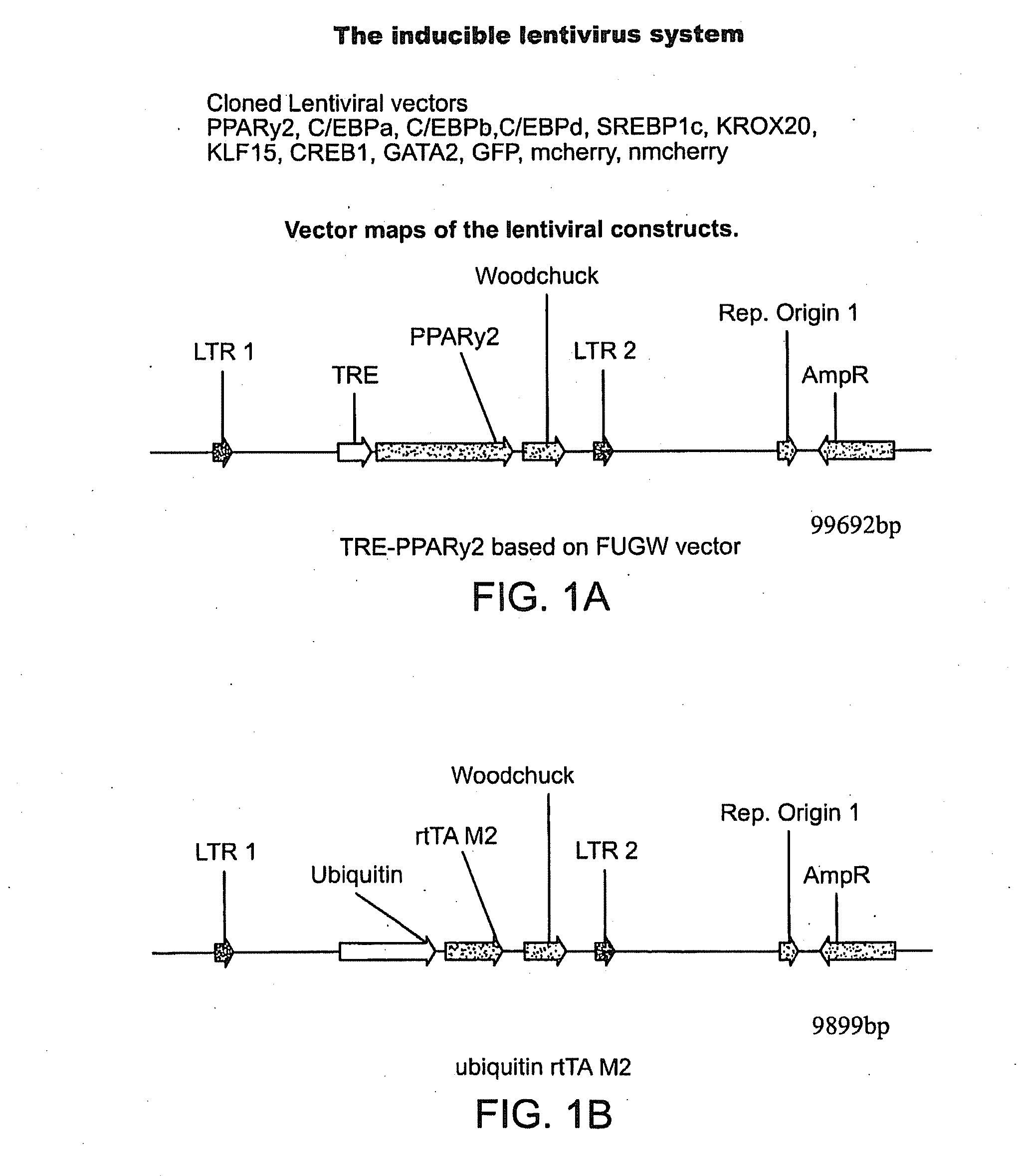
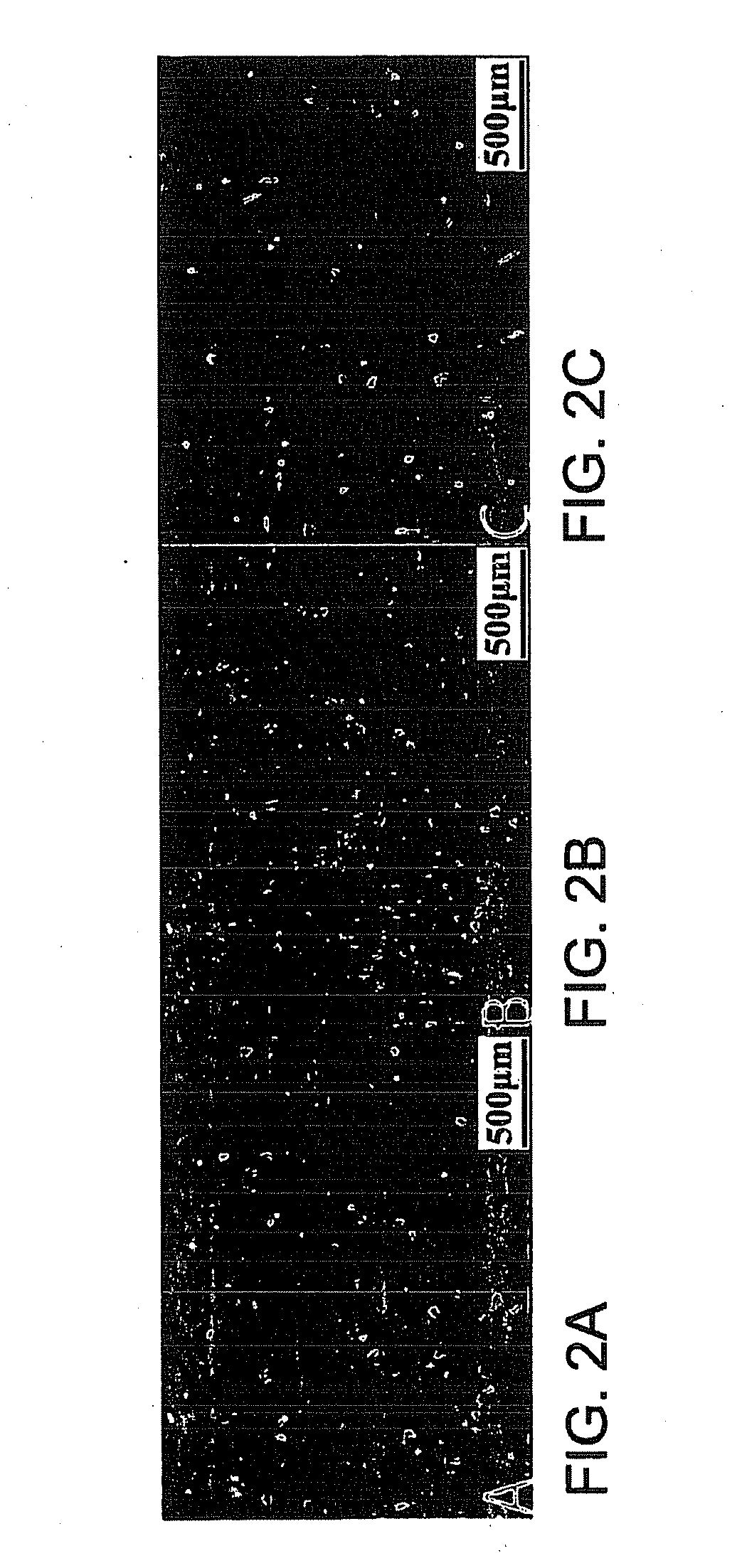
[0191]Known regulators of adipogenesis were cloned into a doxycycline-inducible lentiviral backbone (e.g. PPARγ2, C / EBPα, C / EBPβ, C / EBPδ, SREBP1c, CREB1, and KROX20) (FIG. 1). To test viral titers human adipocyte-derived mesenchymal stem cells (ADMSCs) and human embryonic stem cells were transduced with enhanced green fluorescent protein (eGFP) and reverse tetracycline-controlled transcriptional activator M2 (rtTA-M2) expressing lentiviruses. Fluoresence microscopy of ADMSCs followed by fluorescence-activated cell sorting (FACS) revealed a transfection efficiency of 93% (FIGS. 2A-2C) with a similar efficiency in human embryonic stem cells.
[0192]The adipogenic potential of lentiviral PPARγ2 overexpression in ADMSCs and human embyronic stem cells was tested. In both cases there was differentiation into adipocytes at high efficiency. Of note, discernible differences in cellular morph...
example 2
Reprogrammed Adipocyte Cells are Useful for Identifying Genetic Alterations Associated with Cardiac Pathology
[0197]The methods of the invention provide for the generation of subject-specific tissues for genetic profiling and other studies through the use of reprogrammed induced pluripotent stem (iPS) cells. iPS cell lines can be generated from patient-derived fibrobasts using methods described by (Park et al., Cell. 2008; 134:877-886; Maherali et al., Cell Stem Cell 3, 340-345). Fibroblasts suitable for reprogramming have been obtained from three different sources: skin punch biopsies, hair follicles, and peripheral blood mononuclear cells (PBMC) fractions from blood draws (FIG. 6B). The cells are transduced with a single doxycycline-inducible lentivirus that transiently expresses all of the four genes needed for fibroblast programming into iPS cells (FIGS. 6A-6D). Vectors and methods for generating induced pluripotent stem cells are known in the art and described, for example, in M...
example 3
PPARγ2 Expression is Sufficient for Adipocyte Differentiation In Vitro from both hEs Cells and ADMSCs
[0202]As described above, human somatic cells can be directly reprogrammed in vitro into a pluripotent embryonic stem cell-like state by introducing a combination of four transcription factors (OCT4, SOX2, and either cMYC and KLF4 or NANOG and LIN28) (Takahashi et al., Nat Protoc, 2007. 2(12):3081-9, Yu et al., Science, 2007. 318(5858): 1917-20, Lowry et al., Proc Natl Acad Sci USA, 2008. 105(8): p. 2883-8). These induced pluripotent stem (iPS) cells are nearly identical to embryonic stem (ES) cells in gene expression, DNA methylation and chromatin modifications (Takahashi et al., Nat Protoc, 2007. 2(12):3081-9, Yu et al., Science, 2007. 318(5858): 1917-20, Maherali supra). Using a retroviral system, human iPS cells have successfully been generated from patients with a variety of genetic diseases, including Parkinson disease, Huntington disease, and type 1 diabetes mellitus. A tetrac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Cell death | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com