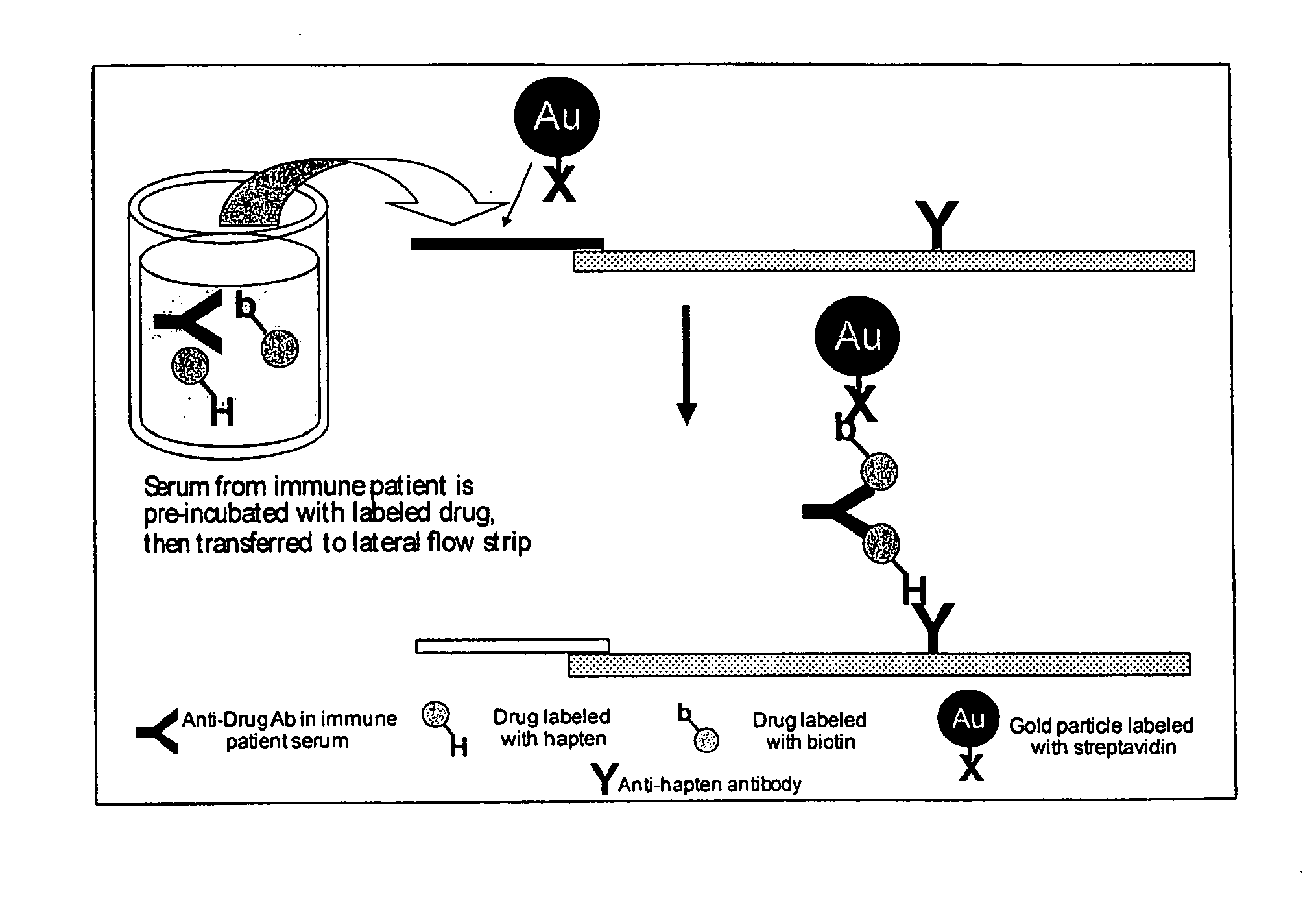
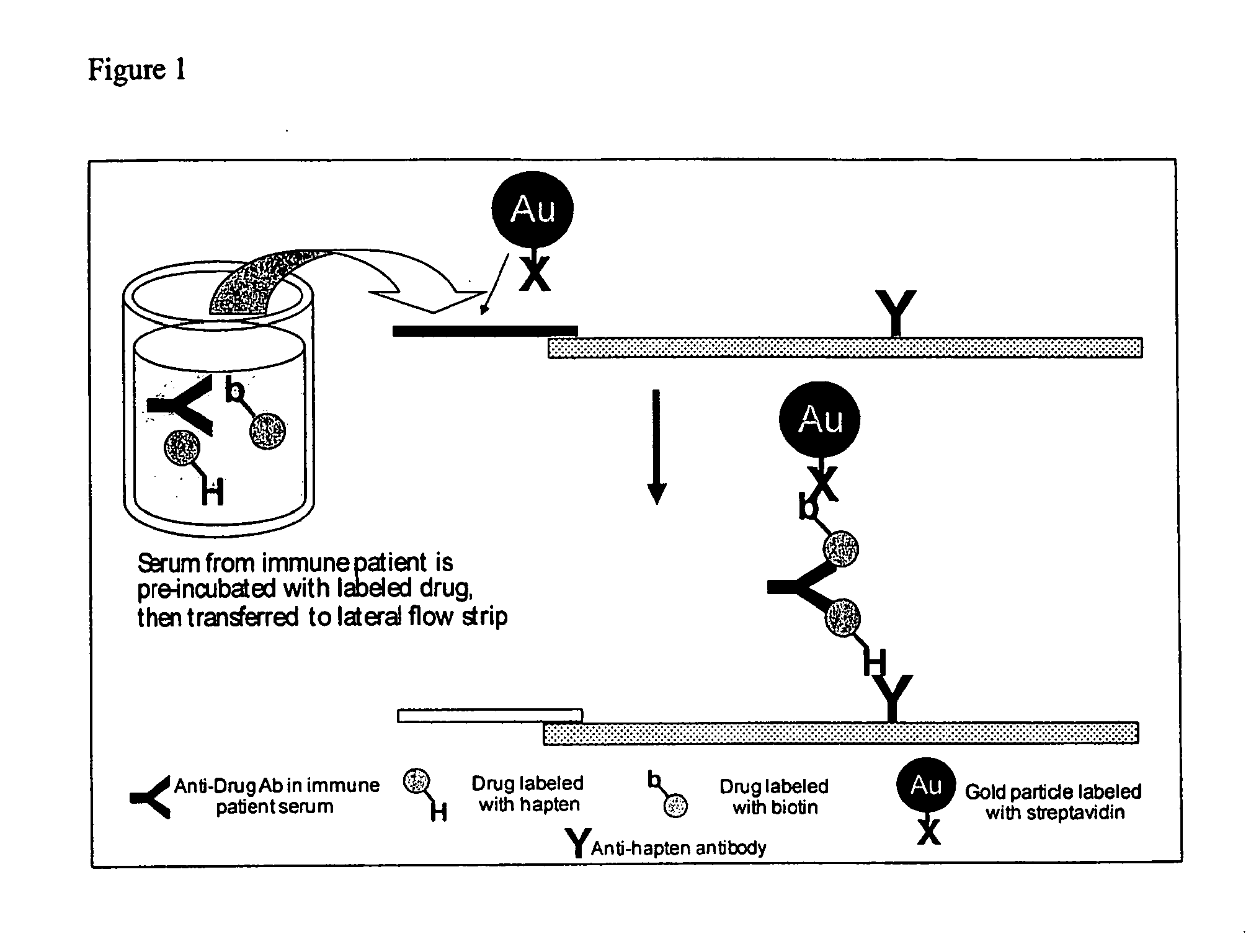
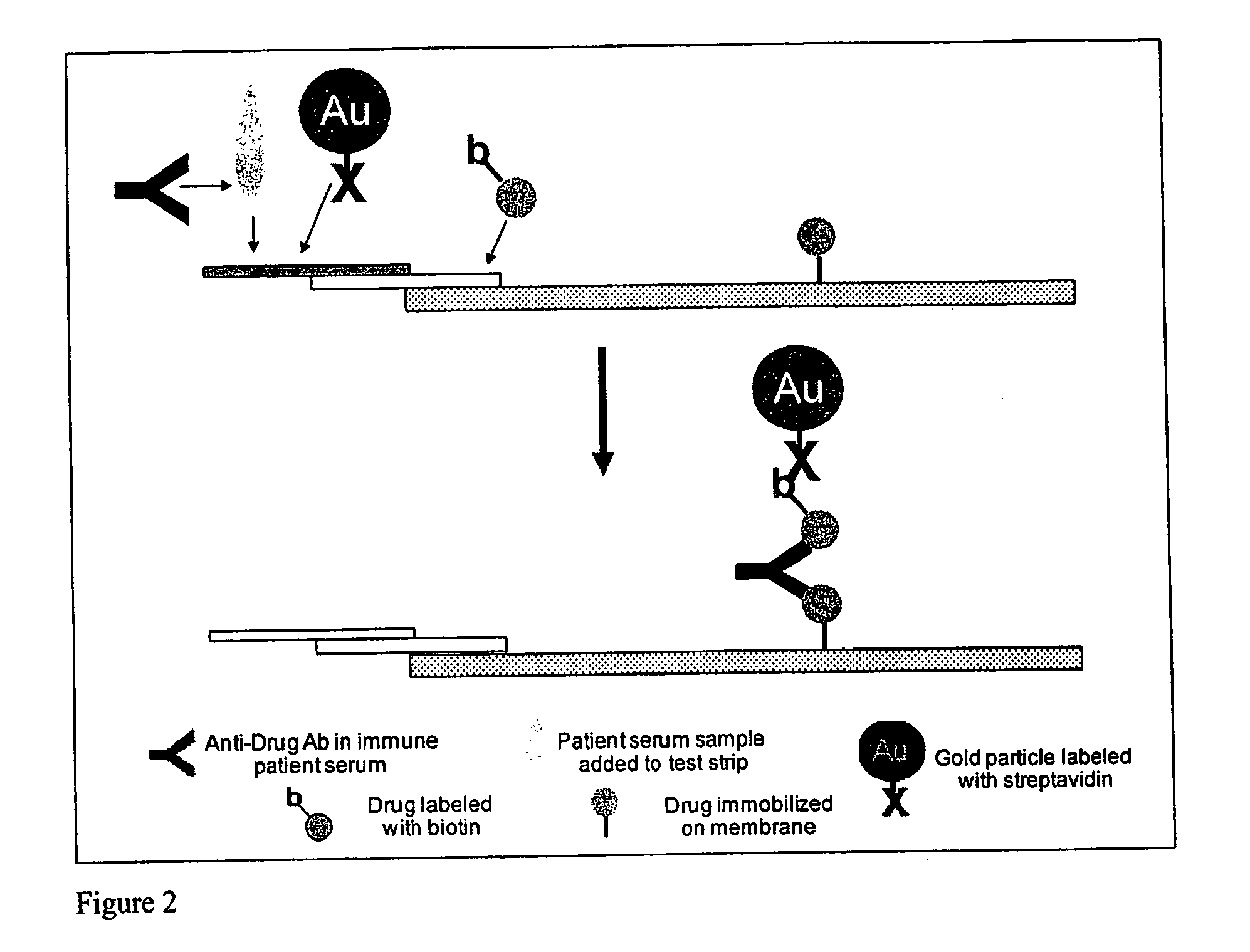
Immunogenicity Assay
a technology of immunogenicity and assay, which is applied in the field of immunogenicity assay, can solve the problems of reducing the ability of conventional methods to detect low titers of antibodies of interest, such as ada, and achieve the effects of improving the likelihood of detecting antibodies, increasing the availability of antigenic epitopes, and facilitating the detection of such antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Biotin and Hapten Conjugation Methods
[0156]The PEG and IgG molecules are used as a generic example for the conjugation of PEG or drug molecules with biotin and haptens such as DNP, digoxigenin, digoxin, fluorescein, thyroxine, THC, morphine, amphetamine, heroin, barbiturates or other tags. Other conjugations of peptide, protein, and DNA / RNA drugs were prepared in a similar manner.
[0157]Method 1 Conjugation of 40 kDa PEG with a biotin (without a linker). PEG-NH2 (MW 40,000, NOF Corporation) (1.33 mg) was dissolved in 1 mL of PBS (BupH phosphate buffered saline from Thermo). N-hydroxysuccinimidobiotin EZ-Link NHS-Biotin from Thermo) (113 μg) was dissolved in 33.3 uL of dimethyl sulfoxide (DMSO). The solution was then added to the PEG solution. The reaction was incubated for 1 hour at room temperature. The product was purified on a desalting column, by ultrafiltration, or dialysis.
[0158]Method 2. Conjugation of 40 kDa PEG with a digoxigenin (without a linker). PEG-NH2 (MW 40,000, NOF C...
example 3
Preparation of Gold-Ab Conjugates
[0187]To a 125 ml flask were added 60 ml of colloidal gold solution (20-80 nm in diameter, O.D. 1.078). The pH of the solution was adjusted to 8-11 by addition of a 0.2 M potassium carbonate solution. To that solution were added 600 ml of conjugated antibody / streptavidin solution (O.D. 0.1-1.5 in sodium borate buffer) while stirring, followed by subsequent addition of 600 ml of bovine serum albumin (20% with sodium azide stabilizer). The mixture was stirred at 20° C. for 20-60 more minutes. The solution remained purple in color and some foaminess was observed. On completion, the stir bar was removed and the reaction mixture was transferred to two 50 ml conical tubes. The material was centrifuged until very little color was observed in the supernatant. The supernatant was removed and 400 ml of 25 mM sodium borate buffer were added to each tube. The contents were mixed thoroughly and the two tubes of material were combined and characterized by UV-Vis.
example 4
Immunogenicity Assay for the Detection of Anti-mouse IgG
[0188]A typical result obtained from a lateral flow assay design is shown in FIG. 3 wherein the drug is a mouse IgG, and the host is a goat that was exposed to the mouse IgG. The LFA format depicted in FIG. 2 was used to generate the dose response curve of FIG. 3 with serial dilutions of the goat antibodies, using an LFA test strip reader (LUR reader, ANP Technologies]. The limit of detection for that assay is 25 ng / mL ADA. The assay design can be used to detect ADA's to any type of therapeutic antibody.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com