Method For Producing An Acellular Dermal Matrix, And Acellular Dermal Matrix Produced By Same
a technology of which is applied in the field of preparing acellular dermal matrix and acellular dermal matrix therefr, can solve the problems of increasing patient's pain, skin tissue may be obtained, and regions can leave new scars, so as to achieve the effect of reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
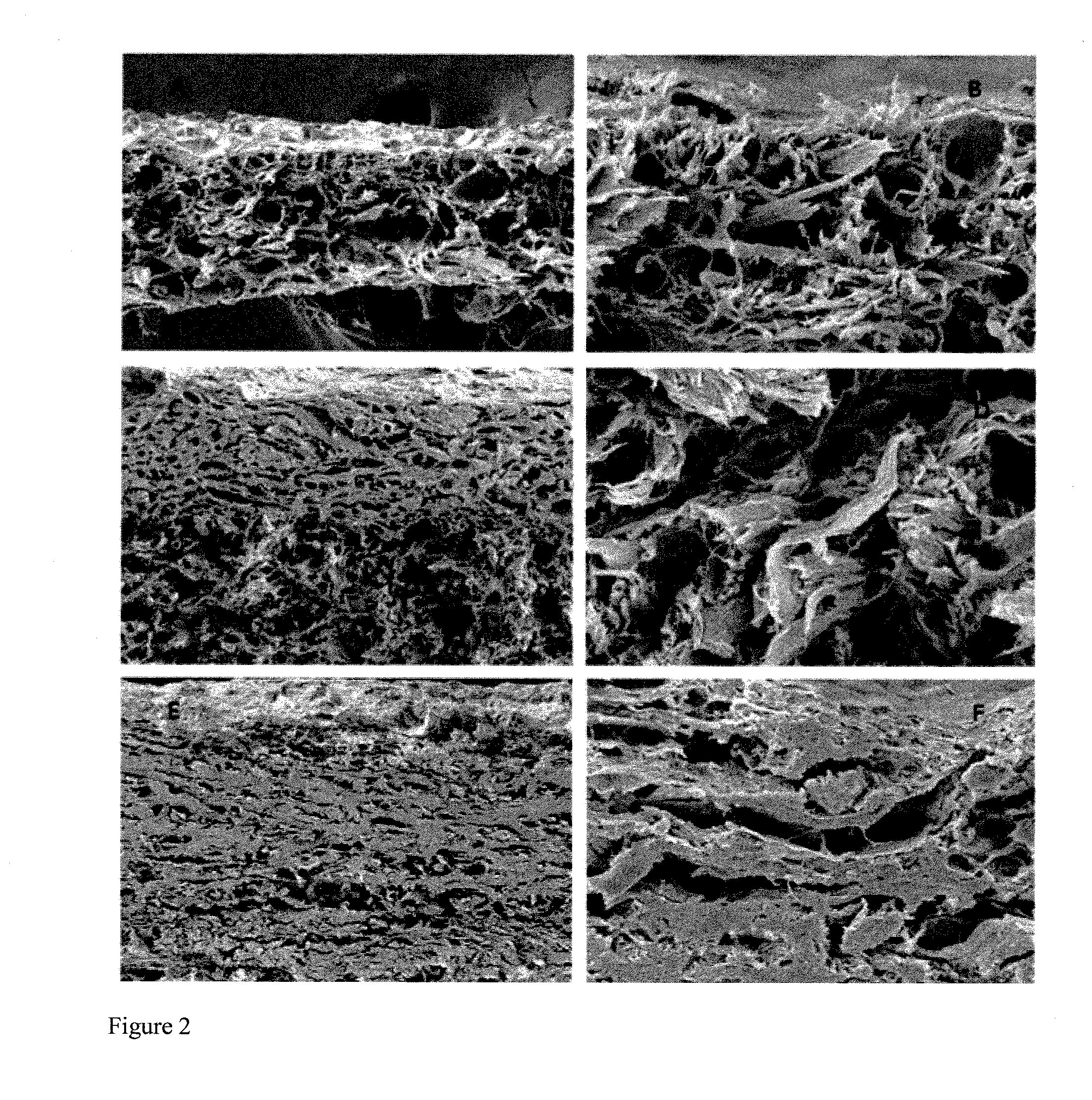
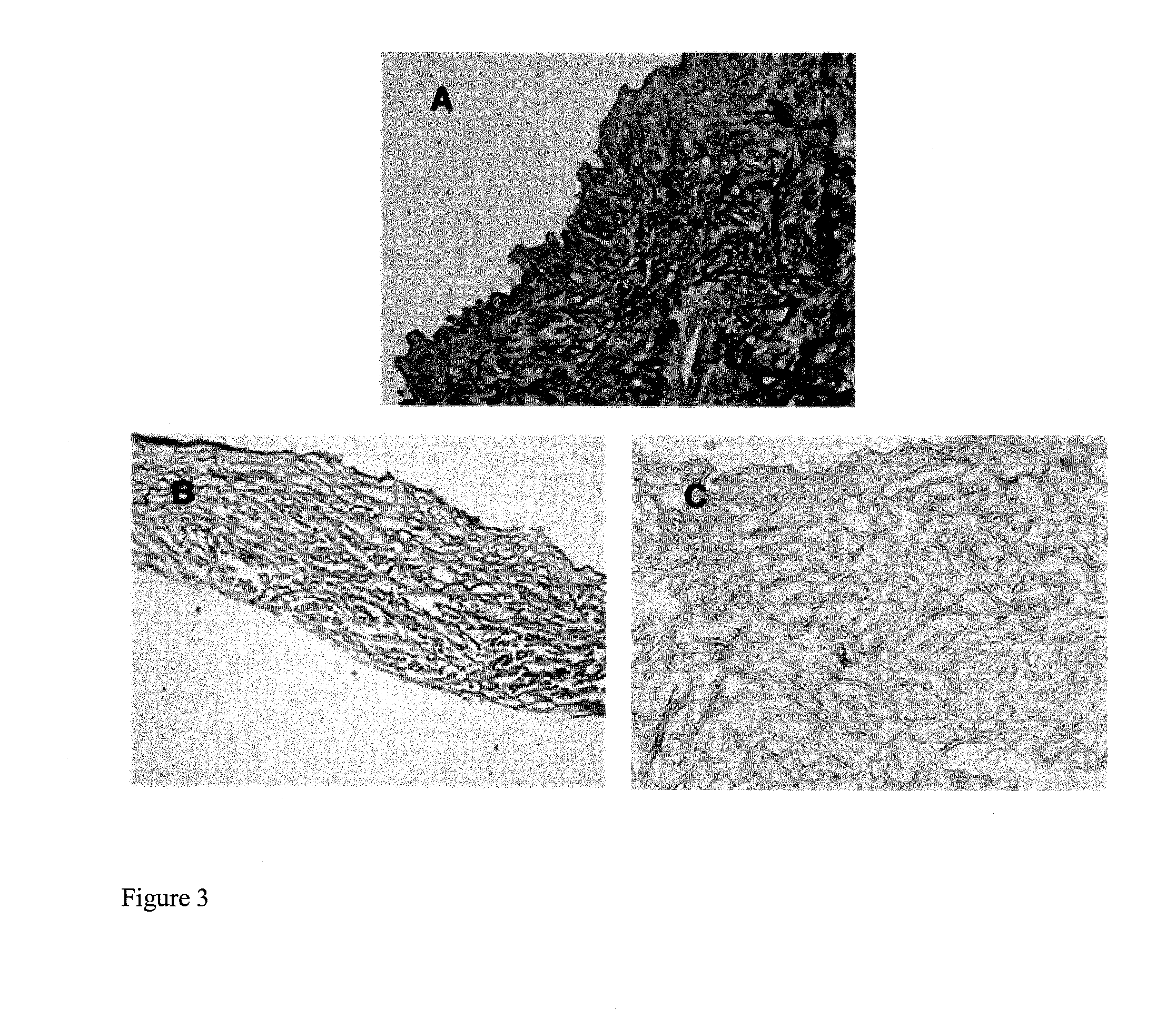
Image
Examples
experimental example 1
Histological Examination
[0066]H&E (hematoxylin & eosin) staining was carried out as follows:
[0067](1) A paraffin block was cut with 4 μm thickness and dried to obtain a paraffin section.
[0068](2) For deparaffinization, after conducting xylene treatment of 5 minutes three times, 100% ethanol treatment of 2 minutes three times, 90% ethanol treatment of 1 minute one time, 80% ethanol treatment of 1 minute one time and 70% ethanol treatment of 1 minute one time, the section was rinsed in running water for 10 minutes.
[0069](3) After staining with hematoxylin for 10 minutes, the section was rinsed in running water for 3 minutes. Then, after staining with eosin for 10 minutes, the section was rinsed in running water until no eosin was detected in the rinse water. After conducting 70% ethanol treatment of 1 second ten times, 80% ethanol treatment of 1 second ten times, 90% ethanol treatment of 1 second ten times, 100% ethanol treatment of 1 minute two times and xylene treatment of 3 minutes...
experimental example 2
Measurement of Degradability by Collagenase
[0080]To evaluate the stability of acellular dermal matrixes of Example and Comparative Examples 1 and 2, the degradability by collagenase was measured as follows:
[0081](1) 25 mg of sample was added to 5 mM TES buffer containing 0.36 mM calcium chloride and mixed well.
[0082](2) 0.1 ml of collagenase (0.1 mg / ml) was added to the sample of step (1) and incubated at 37° C. for one day with stirring.
[0083](3) 4.0 mM L-leucine standard solution was serially diluted and treated with ninhydrin color reagent. A standard curve was prepared by measuring absorbance at 570 nm (VERSA max, Molecular Device, USA).
[0084](4) The sample of step (2) was treated with ninhydrin color reagent and absorbance at 570 nm was then measured.
[0085](5) The amount of released L-leucine from each sample was calculated by using the L-leucine standard curve of step (3).
[0086]The above calculated L-leucine release amount is represented in FIG. 4. As can be seen from FIG. 4, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com