Iodine-sulfur cycle for nuclear hydrogen production with improved thermo-chemical efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034]Hereinafter, certain embodiments of the present invention will be described in detail with reference to the attached drawings.
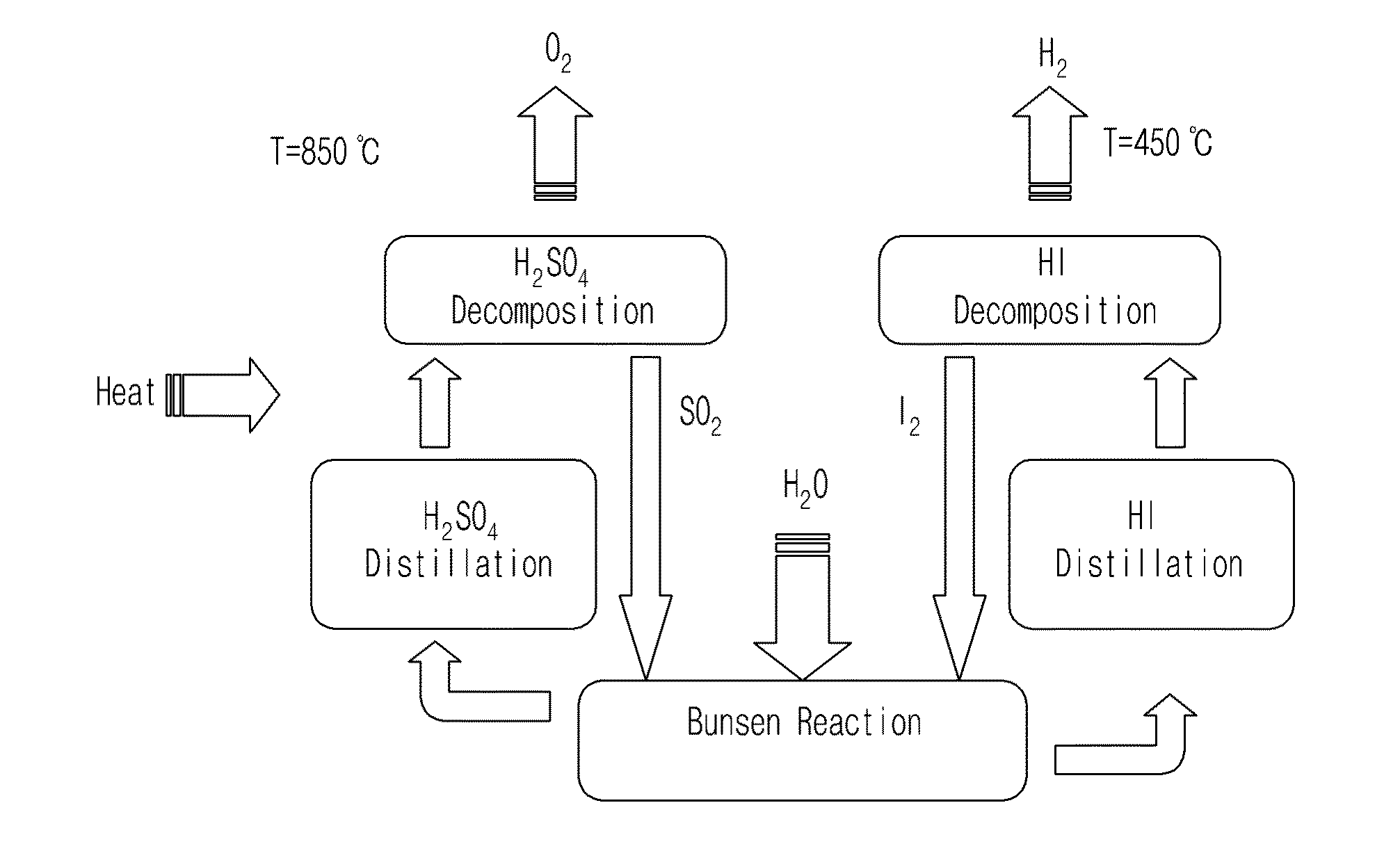
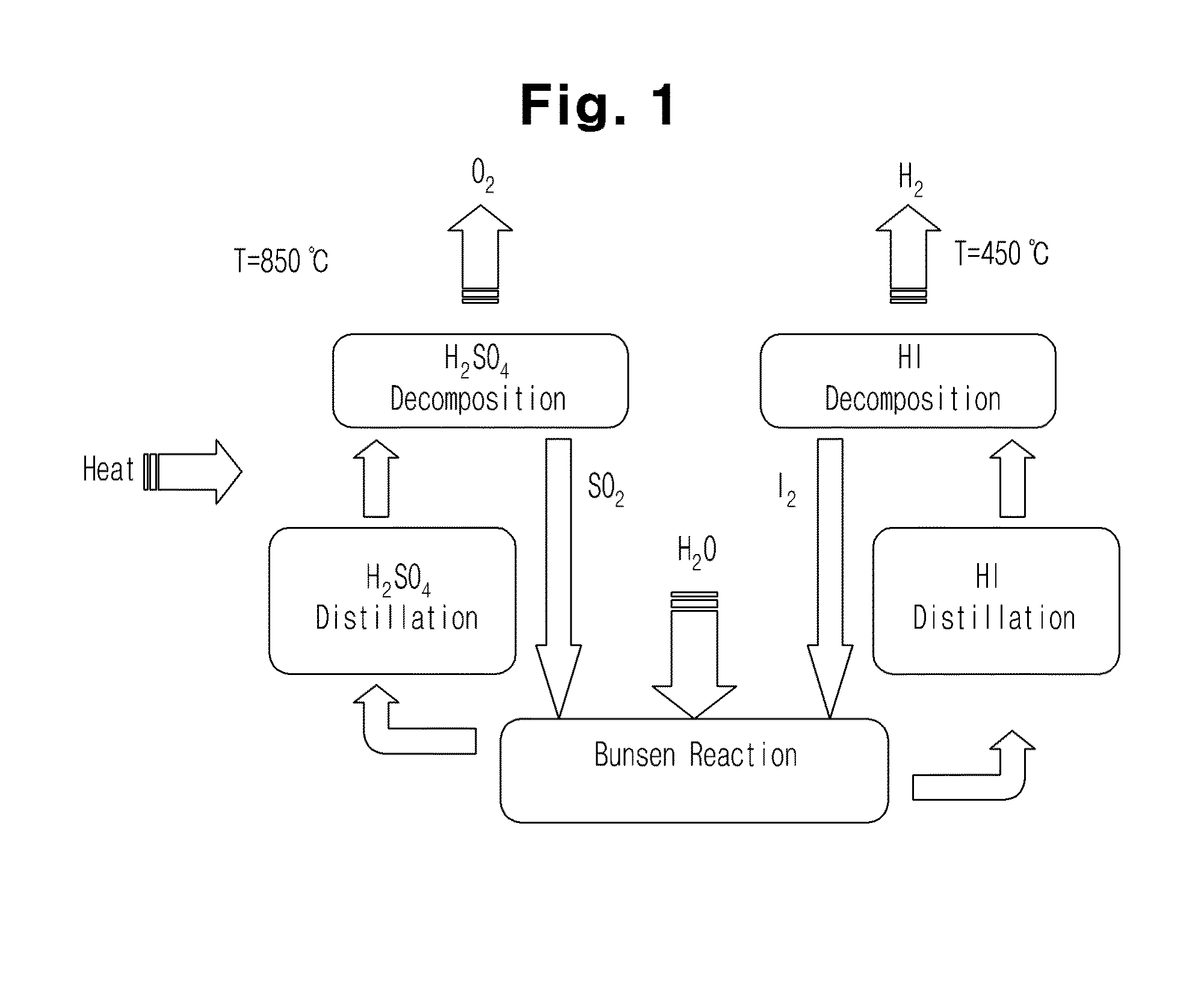
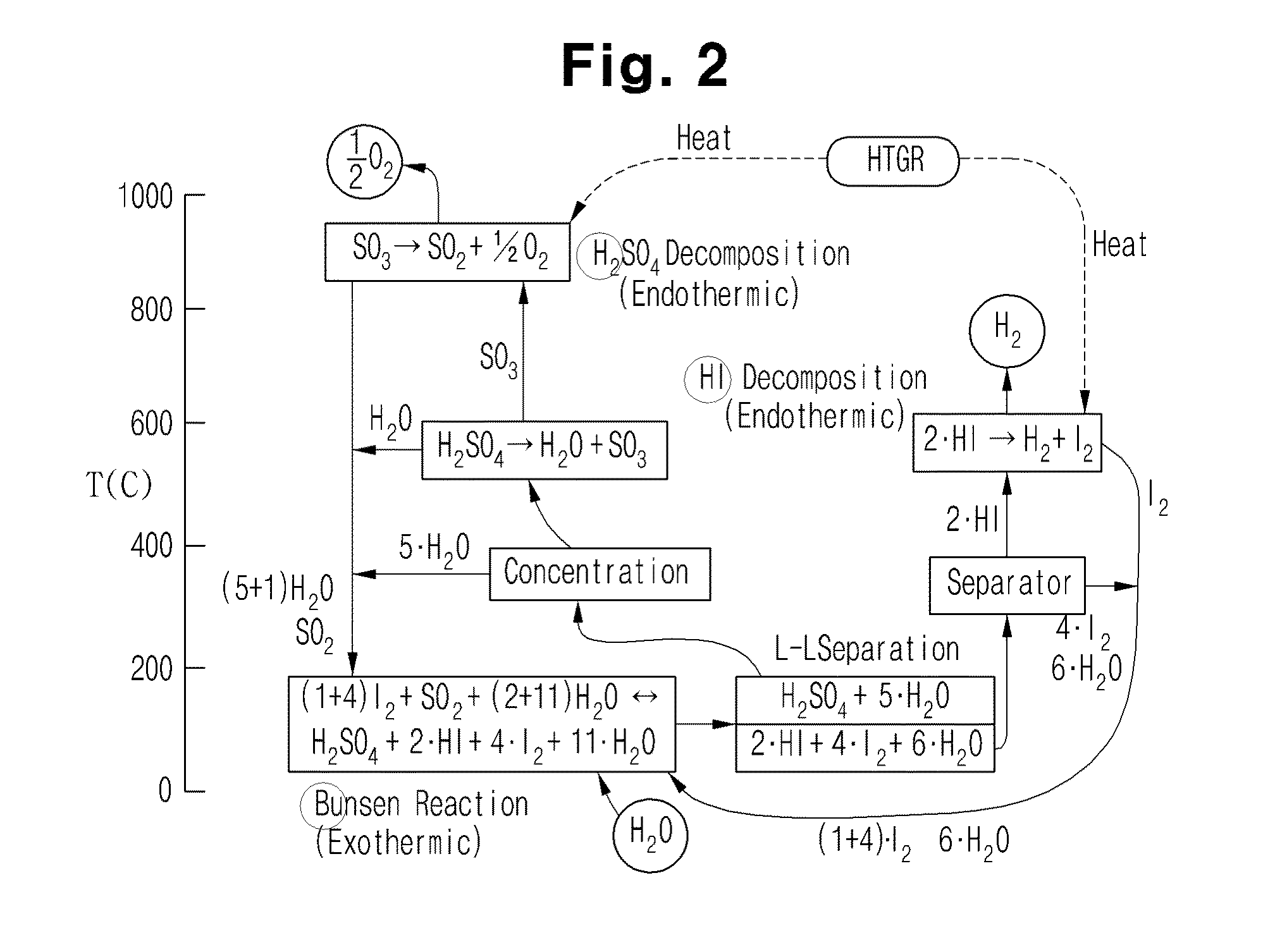
[0035]The present invention provides an iodine-sulfur cycle for nuclear hydrogen production, including a Bunsen reaction process including a liquid-liquid phase separation process, a hydrogen iodide decomposition process and a sulfuric acid decomposition process, wherein sulfur dioxide, iodine, and water, which are reactants necessarily used to conduct the Bunsen reaction process, mix with excess water and excess iodine and then react with each other at an operating temperature of 330˜350K (57˜77° C.) as represented by the following Reaction Formula 1.
(5˜7)·I2+SO2+(13˜15)·H2O[2HI+4·I2+(6˜8)·H2O]+[H2SO4+5·H2O] [Reaction Formula 1]
[0036]In the Reaction Formula 1, based on the composition ratio of reactants at an optimal operating point at which the minimum amount of excess water required to be supplied such that the Bunsen reaction smoothly proceeds in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com