Lipase inhibitors
a technology of lipase inhibitors and inhibitors, applied in the field of lipase inhibitors, can solve the problems of insufficient energy mobilization of atgl-deficient animals, affecting the rate of atgl, cardiac dysfunction and premature death, etc., and achieve the effect of inhibiting atgl and lowering blood ffa values
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods
[0111]cDNA cloning and transient expression of recombinant His-tagged proteins in COS-7 cells and 3T3-L1 adipocytes.
[0112]The coding sequences of mouse ATGL and HSL are amplified by PCR from cDNA prepared from mRNA of mouse white adipose tissue by reverse transcription. The open reading frame, flanked by KpnI / XhoI sites for ATGL and HSL were cloned into the eucaryotic expression vector pcDNA4 / HisMax (Invitrogen). Transfection of COS-7 cells was performed with Metafectene™ (Biontex) according to the manufacturer's description. The PCR primers used to generate these probes were as follows.
ATGL forward5′-TGGTACCGTTCCCGAGGGAGACCAAGTGGA-3′,ATGL revers5′-CCTCGAGCGCAAGGCGGGAGGCCAGGT-3′.HSL forward5′-TGGTAGGT-ATGGATTTACGCACGATGACAGA-3′,HSL revers5′-CCTCGAGCGTTCAGTGGTGCAGCAGGCG-3′.
Construction of the Recombinant Adenovirus for ATGL Expression (ATGL-Ad) and Infection of 3T3-L1 Cells:
[0113]The recombinant adenovirus coding for mouse ATGL is prepared by cotransfection of the shuttle plas...
example 2
Compound Chemistry
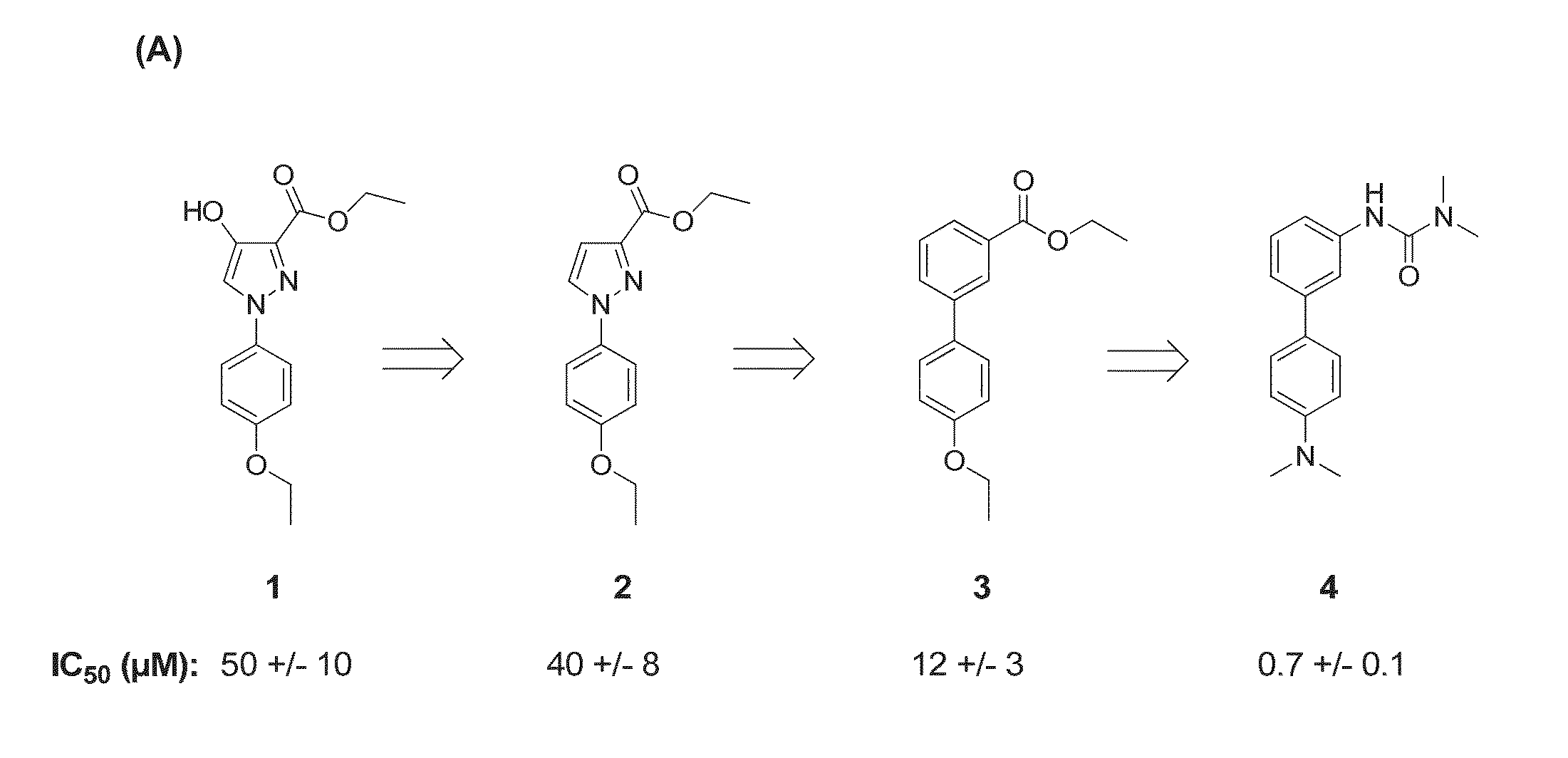
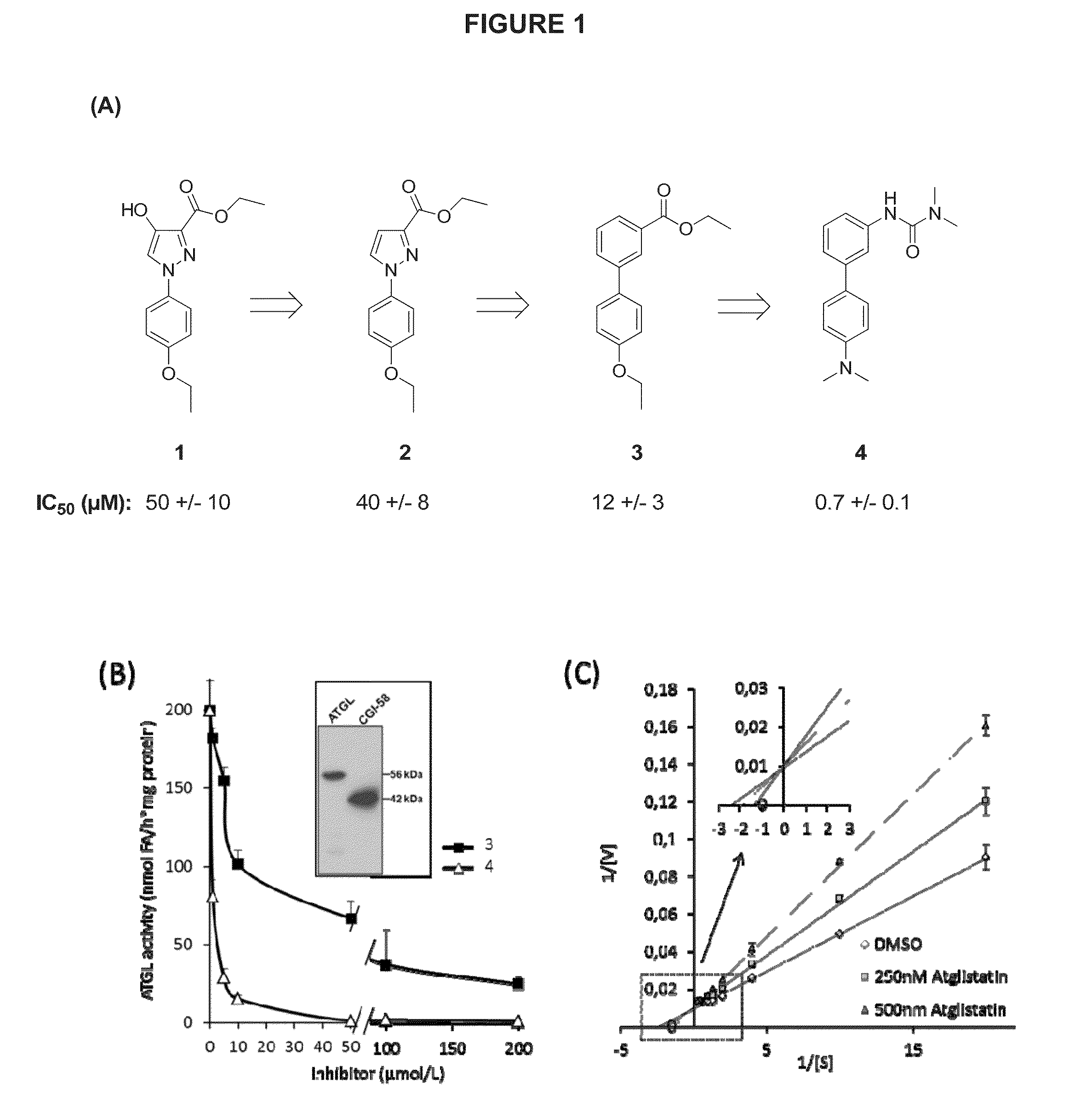
[0161]Compound 1 (FIG. 1A) inhibits the activity of recombinant ATGL (IC50=50 μM). The HO-group in 4-position could be an anchor point for path II metabolism. In order to test the effect of the HO-group in the 4-position on the inhibitory effect and toxicity, compound 2 was prepared by Ullmann-arylation of ethyl pyrazol-3-carboxylate. This compound inhibited ATGL with an IC50 of =40 μM. Both compounds showed some limited toxicity in in vitro tests. The pyrazole moiety was therefore targeted for replacement. Next, a series of compounds were prepared in which the pyrazole was replaced by other aromatic and heteroaromatic rings keeping the 1,3-arrangement of the 4-ethoxyphenyl- and ethoxycarbonyl-substituents constant.
[0162]Using this strategy, biphenyl compound 3 (IC50=12 μM, FIG. 1) emerged as a suitable scaffold. Next, the role of the ethoxy substituent in the bottom ring was investigated. A series of compounds prepared by Pd-catalyzed Suzuki-coupling of different ...
example 3
Kinetic Studies of Inhibitor Action
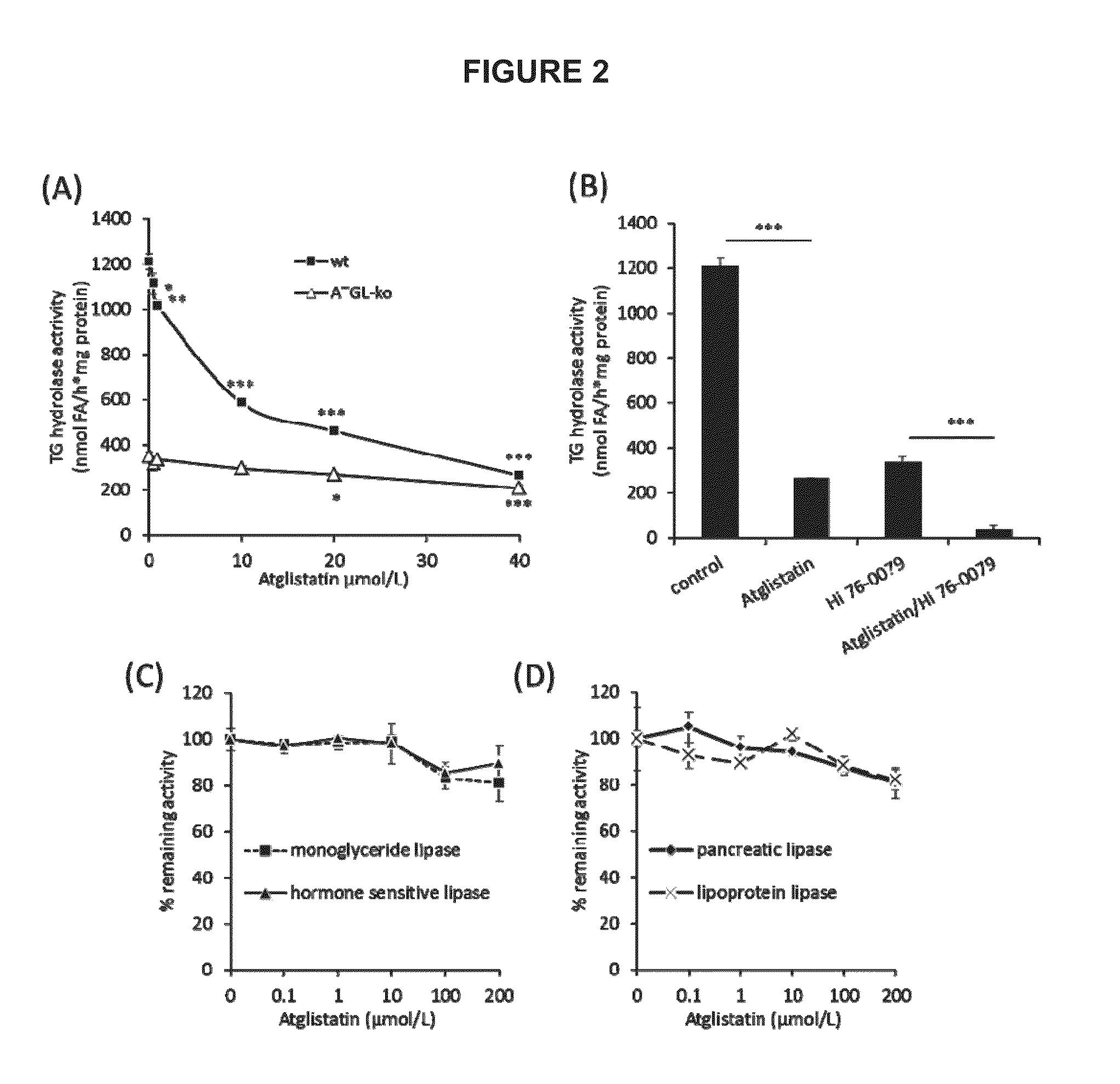
[0163]To investigate the mechanism of Atglistatin-mediated ATGL inhibition, kinetic studies were performed by varying substrate and inhibitor concentrations. Kinetic analysis revealed an increase in the apparent Km values and unchanged Vmax (FIG. 1C) indicating that Atglistatin acts as a competitive inhibitor. Based on the increase of Km values determined in three independent experiments and using non-linear regression analysis (SigmaPlot 12.0), a Ki value of 0.5+ / −0.2 μM was calculated. However, it has to be considered that this is a relative value, since the TG substrate is not water-soluble and consequently only partially available for the reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com