Thermosensitive Nanoparticle Formulations and Method of Making The Same
a technology of thermosensitive nanoparticles and formulations, which is applied in the field of thermosensitive liposome formulations, can solve the problems of limited techniques, long-term stability of drug products, and achieve the effects of long-term stability and significant impact on the storage temperatur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Doxorubicin Loaded Temperature-Sensitive Liposomes by NH4+-Loading
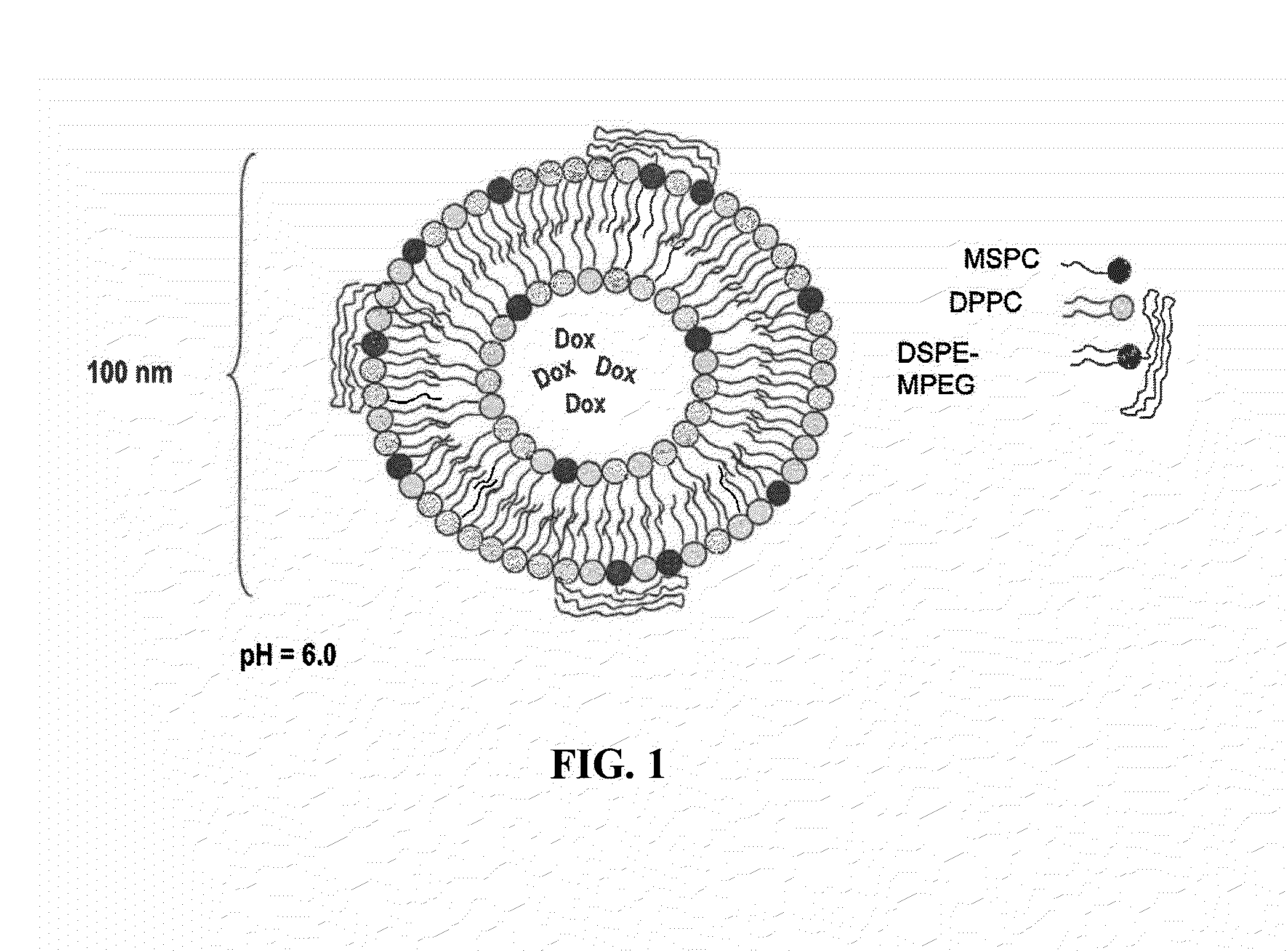
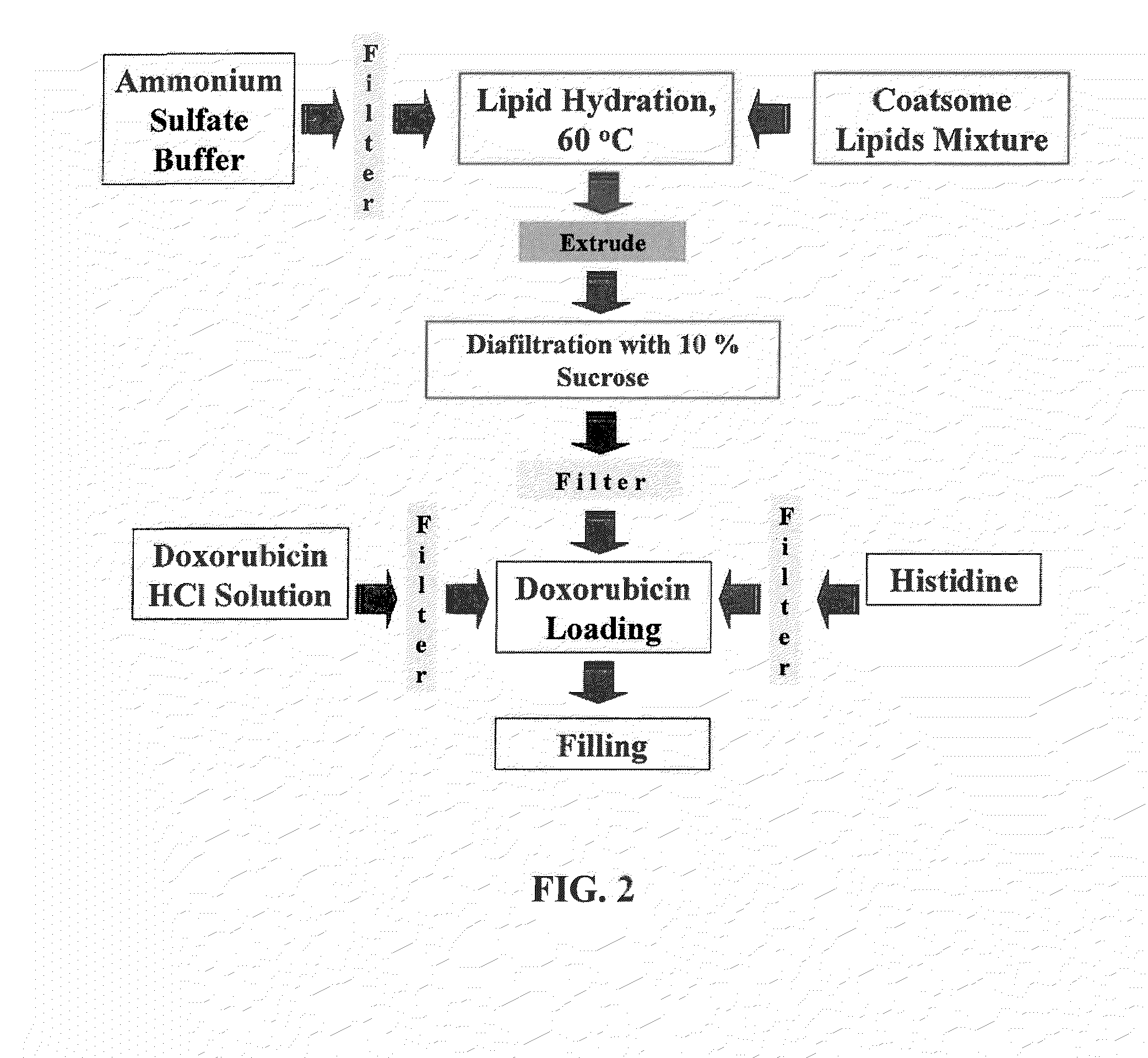
[0092]Liposomes containing 1,2-dipalmitoyl-sn-glycero-3-phosphatidyl choline (DPPC), which comprises 86% (mole %) of the liposome membrane; 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-polyethylene glycol 2000 (DSPE-mPEG), at approximately 4% (mole %); and 1-stearoyl-2-hydroxy-sn-glycero phosphatidyl choline (MSPC) at approximately 10% (mole %) are prepared by the following technique: The appropriate lipid composition is first hydrated in 200 mM ammonium sulfate buffer, forming multi-lamellar liposomes. Small uni-lamellar liposomes are then formed by extrusion through 80 nm filters to form approximately 100 nm spheres in 200 mM ammonium sulfate buffer.
[0093]The liposomes prepared in the previous step were then subjected to a dialysis or diafiltration step exchanging the ammonium sulfate that is external to the liposome with a 10% sucrose solution, forming an ammonium concentration gradient across t...
example 2
Preparation of pH-Loaded Temperature Sensitive Doxorubicin Liposomes
[0094]Liposomes with doxorubicin loaded using a pH gradient are prepared according to the method described in WO 2007 / 024826, Liposomes containing 1,2-dipahnitoyl-sn-glycero-3-phosphatidyl choline (DPPC), which comprises 86% (mole %) of the liposome membrane; 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-polyethylene glycol 2000 (DSPE-mPEG), at approximately 4% (mole %); and 1-stearoyl-2-hydroxy-sn-glycero phosphatidyl choline (MSPC) at approximately 10% (mole %) are prepared by the following technique: The appropriate lipid composition is first hydrated in 300 mM citrate buffer (pH=4), forming multi-lamellar liposomes. Small uni-lamellar liposomes are then formed by extrusion through 80 nm filters to form approximately 100 nm spheres in 300 mM citrate buffer.
[0095]A 500 mM sodium carbonate solution is then added to the liposomes prepared in the previous step, increasing the external solution to a pH of ˜7.5. It...
example 3
Final Product Characterization Methods
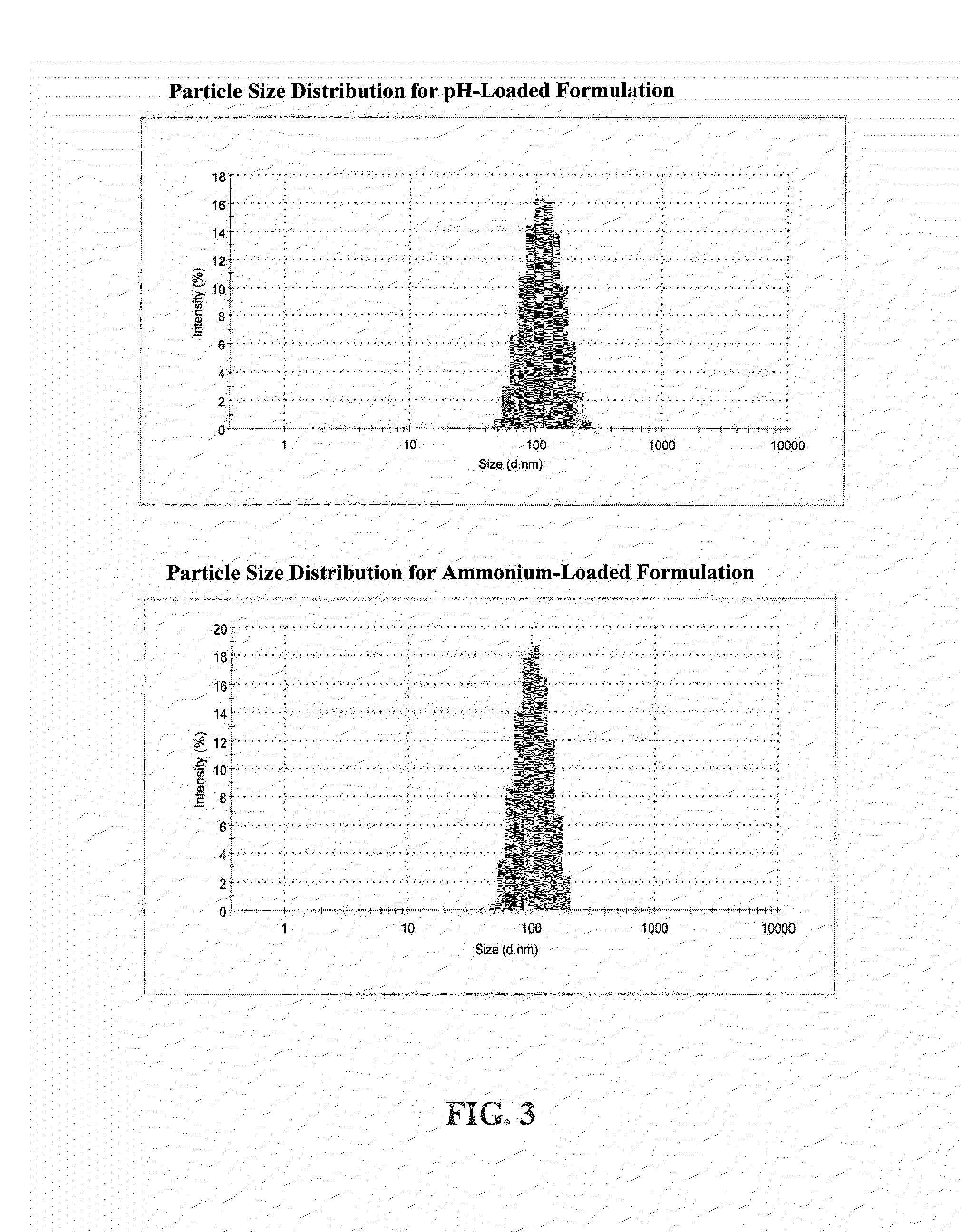
[0097]The final product is characterized for total doxorubicin content, doxorubicin degradation products, pH, osmolality, particle size distribution, MSPC content, DPPC content, DSPE-mPEG content, % encapsulated doxorubicin, drug release at 37° C., and drug release at 41° C. to effectively complete assessment of the product. The target total doxorubicin content is between about 1.8 to about 2.2 mg / mL. The drug encapsulation was typically greater than 90%, and showed limited release, e.g. 80%, at 41.0° C. The volume averaged particle size of the liposomes as measured by dynamic light scattering is between about 50 to about 150 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com