Process for recovering alkali metals and sulfur from alkali metal sulfides and polysulfides
a technology of which is applied in the field of process for recovering alkali metals and sulfur from alkali metal sulfide and polysulfide, can solve the problems of air pollution, difficult removal, and technical problems that remain to be solved, and achieves the effects of reducing the solubility of sulfur, reducing the difficulty of removal, and improving the effect of specific gravity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
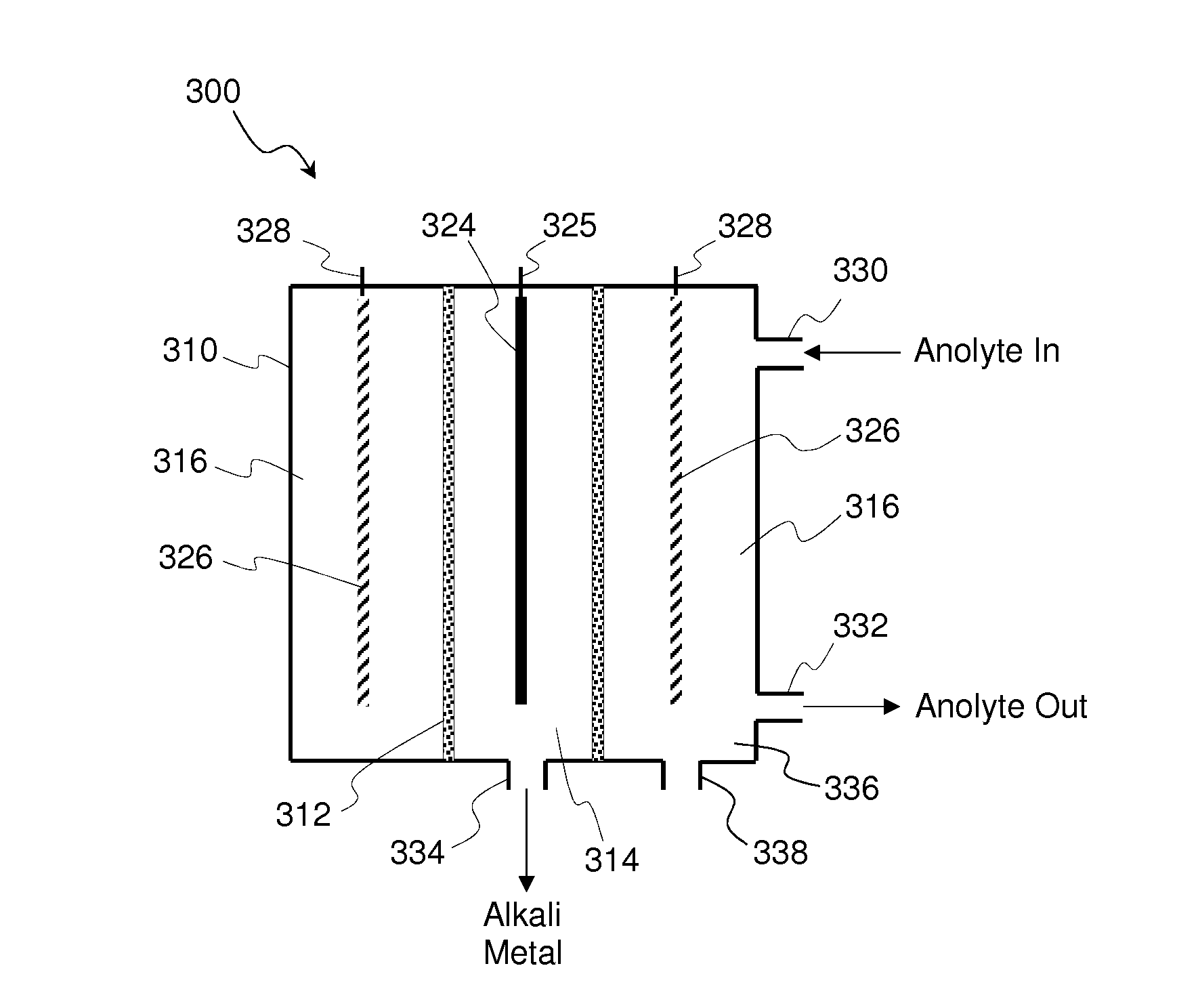
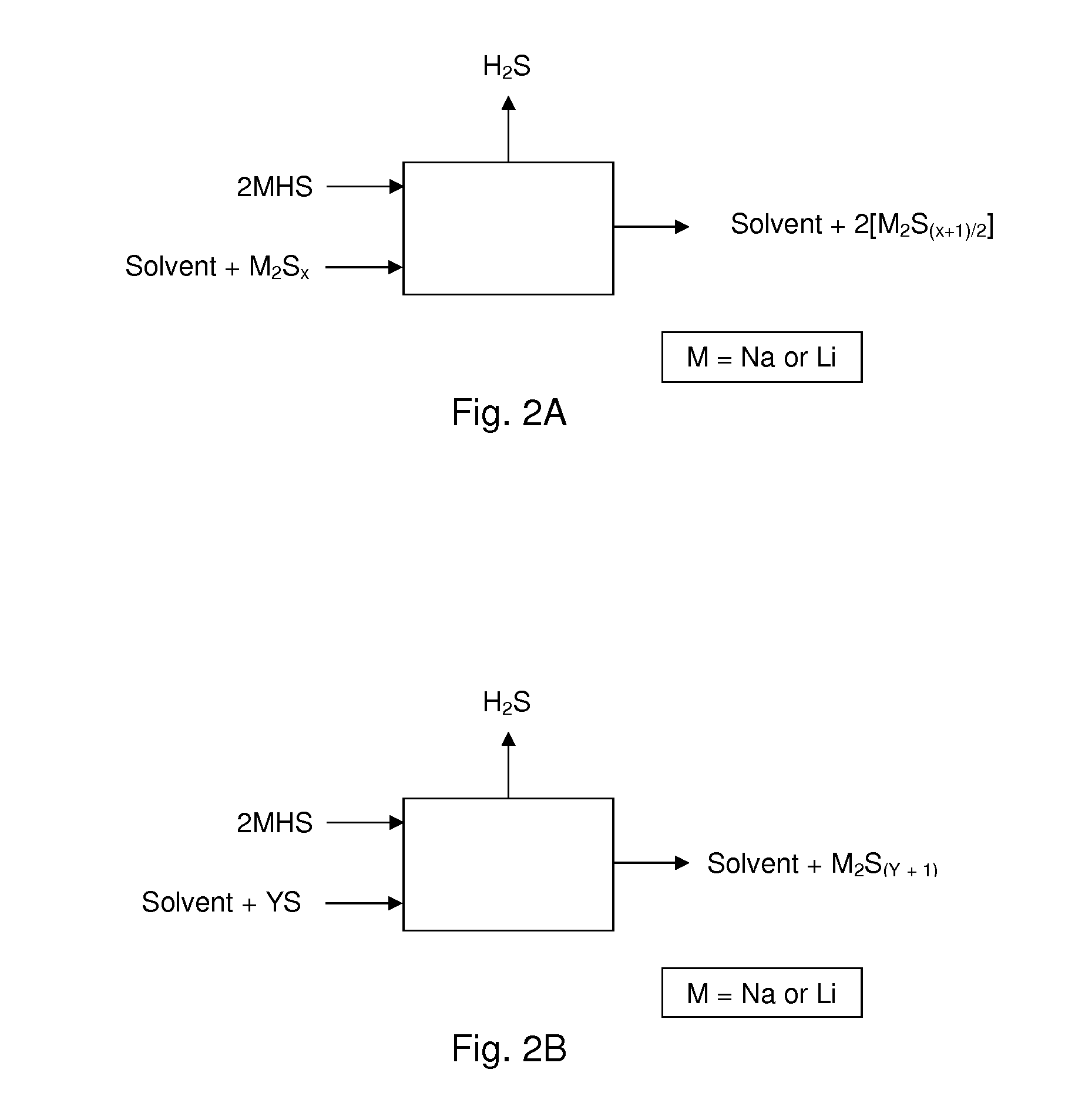
[0048]The present embodiments of the present invention will be best understood by reference to the drawings, wherein like parts are designated by like numerals throughout. It will be readily understood that the components of the present invention, as generally described and illustrated in the figures herein, could be arranged and designed in a wide variety of different configurations. Thus, the following more detailed description of the embodiments of the methods and cells of the present invention, as represented in FIGS. 1 through 4, is not intended to limit the scope of the invention, as claimed, but is merely representative of present embodiments of the invention.
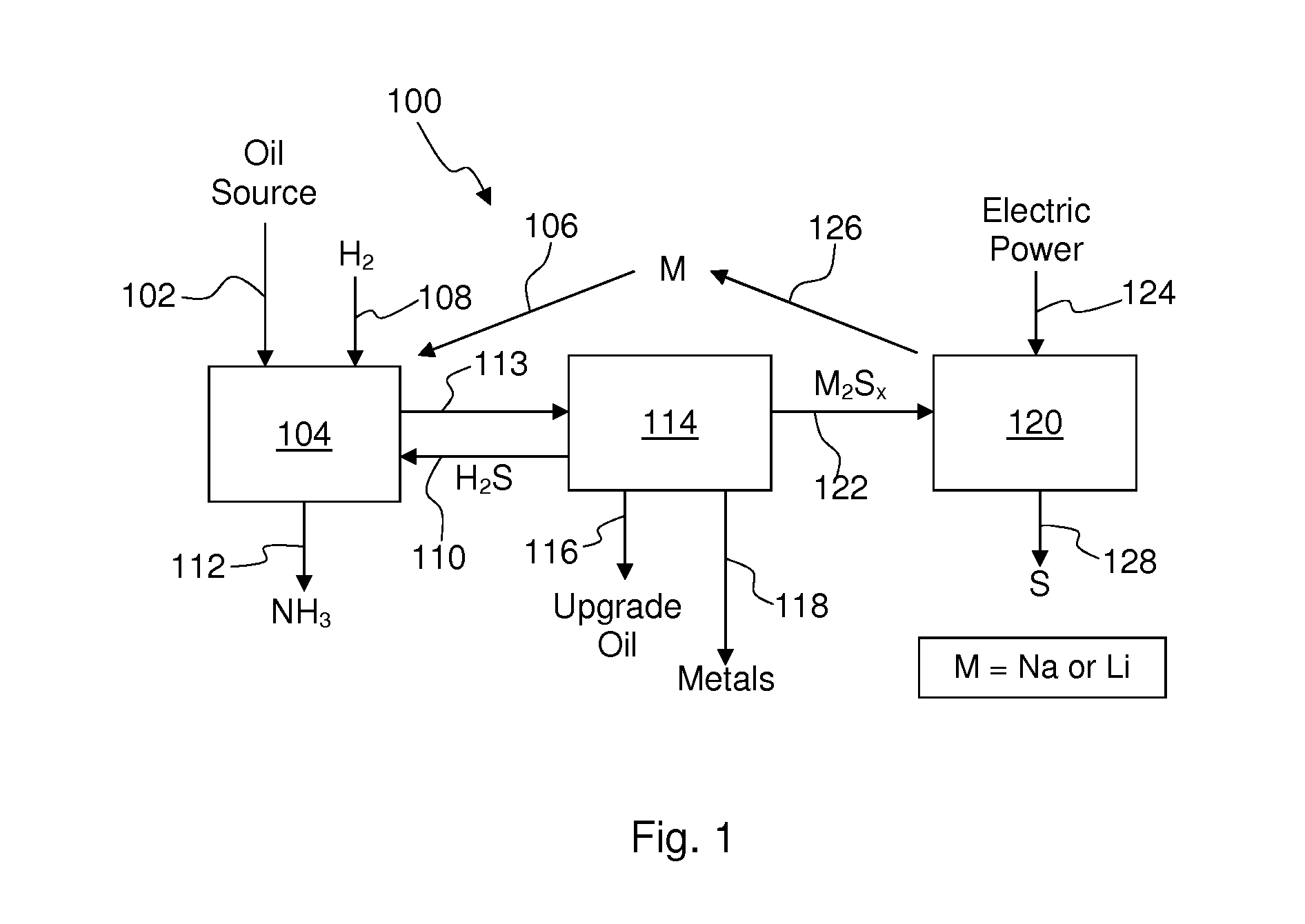
[0049]The overall process is shown schematically in FIG. 1 of one non-limiting embodiment for removing nitrogen, sulfur, and heavy metals from sulfur-, nitrogen-, and metal-bearing oil sources using an alkali metal and for regenerating the alkali metal. In the process 100 of FIG. 1, an oil source 102, such as high-sulfur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conductive | aaaaa | aaaaa |
| operating temperature | aaaaa | aaaaa |
| electric current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com