Topical composition comprising a film-forming polymer for delivering an active ingredient to skin
a technology of film-forming polymer and active ingredient, which is applied in the direction of drug compositions, aerosol delivery, immunological disorders, etc., can solve the problems of minor release of active ingredient incorporated in the composition, and the drawback of the film-forming composition disclosed in the literatur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0039]In the present composition, the film-forming polymer may be selected from the group consisting of cellulose derivatives, acrylic polymers, acrylic copolymers, methacrylate polymers, methacrylate copolymers, polyurethanes, polyvinylalcohol or a derivative thereof such as polyvinylacetate, silicone polymers and silicone copolymers, and copolymers thereof.
[0040]When the film-forming polymer is a cellulose derivative, it may be selected from the group consisting of ethyl cellulose, methyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose.
[0041]When the film-forming polymer is an acrylic polymer, it may be selected from the group consisting of methyl methacrylate and butyl methacrylate copolymer, ethyl acrylate and methyl methacrylate copolymer, acrylate and ammonium methacrylate copolymer type A and type B, and acrylates / octylacrylamide copolymer.
[0042]The film-forming polymer may suitably be present in an amount of 5-20% w / w such as 10-15% ...
example 1
Compositions
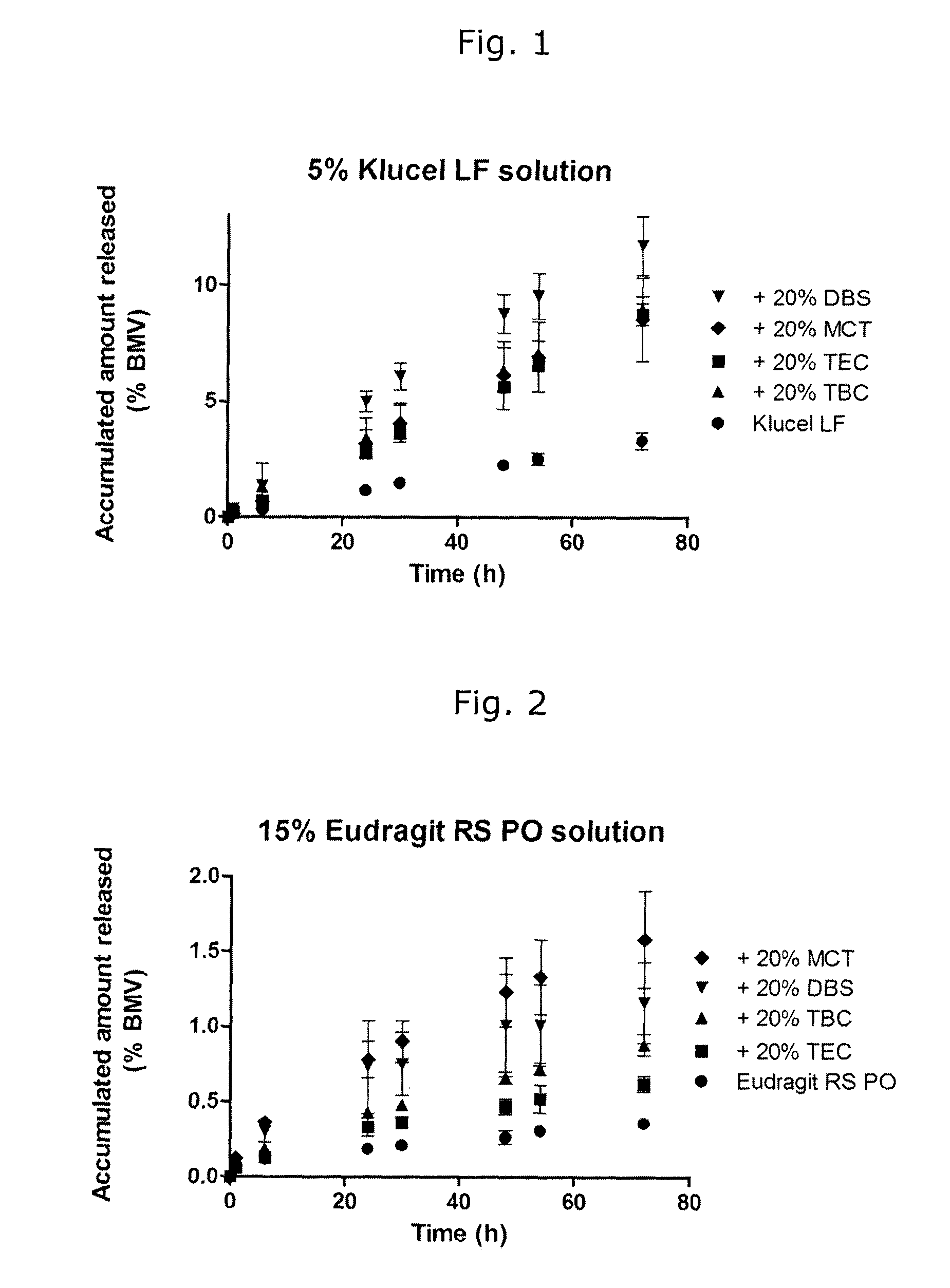
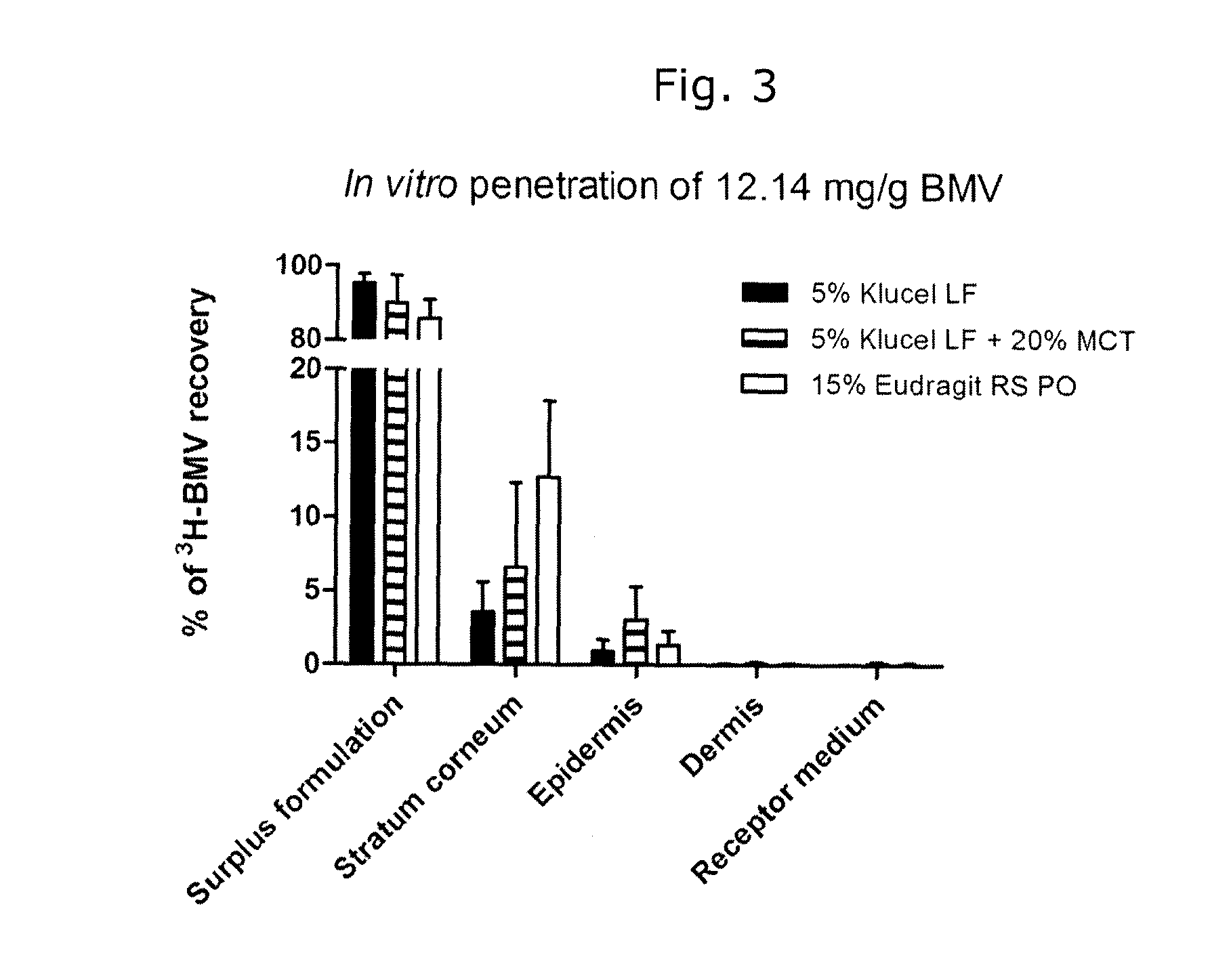
[0073]Reference compositions were prepared including the following ingredients.
PlasticizerOilSolventPolymerTECTBCDBSPEGMCTEthanolKlucel LF 5%XXXXXXEudragit E 15%XXEudragit RS 15%XXXXXDermacryl 79 10%XXXDermacryl 79 + KlucelXXLFTEC: triethyl citrateTBC: tributyl citrateDBS: dibutyl sebacatePEG: polyehtylene glycol 400MCT: medium chain triglycerides
[0074]The content of plasticizer and / or oil in the compositions was 20% by weight of the dry film-forming polymer. In addition, 1.2% by weight of betamethasone valerate (1% by weight betamethasone) was added to the compositions.
[0075]To prepare the compositions, BMV, plasticizer or oily release-enhancing agent (MCT) were dissolved in the solvent by stirring for 1-2 hours. The film-forming polymer was added slowly with stirring, and the resulting mixture was stirred overnight to complete the dissolution of the polymer.
example 2
Compositions
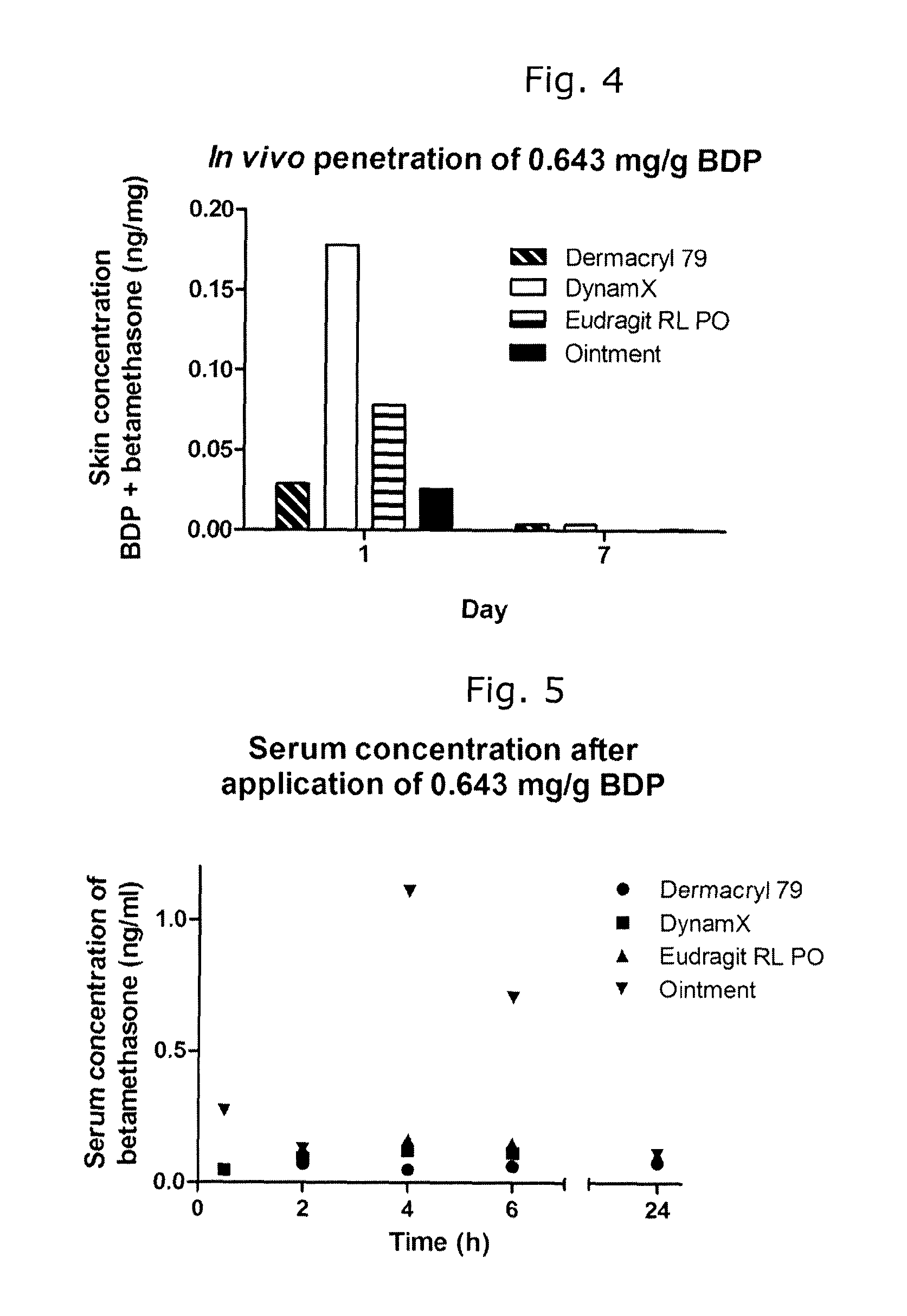
[0076]
Ingredients (mg / g)01A02A03A04ABetamethasone diproprionate12.8612.8612.8612.86Acrylates / octylacrylamide100100100100copolymer (Dermacryl 79)PPG-11 stearyl ether (Arlamol E)202020Polysorbate 8022Tributyl citrate20Ethanol, anhydrous887.14867.14865.14845.14
[0077]Composition 04A is a composition according to the invention, while compositions 01A, 02A and 03A are reference compositions.
[0078]To prepare the compositions, BDP, plasticizer, oily release-enhancing agent (Arlamol E) (as appropriate) and polysorbate 80 (as appropriate) were dissolved in the solvent by stirring for 1-2 hours. The film-forming polymer was added slowly with stirring, and the resulting mixture was stirred overnight to complete the dissolution of the polymer.
Compositions According to the Invention
[0079]
Ingredients (mg / g)05A06A06PBetamethasone valerate12.1412.14Eudragit RS PO150150150Medium chain triglyceride306060Tributyl citrate303030Ethanol, anhydrous777.9747.9760
[0080]To prepare the compositions,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com