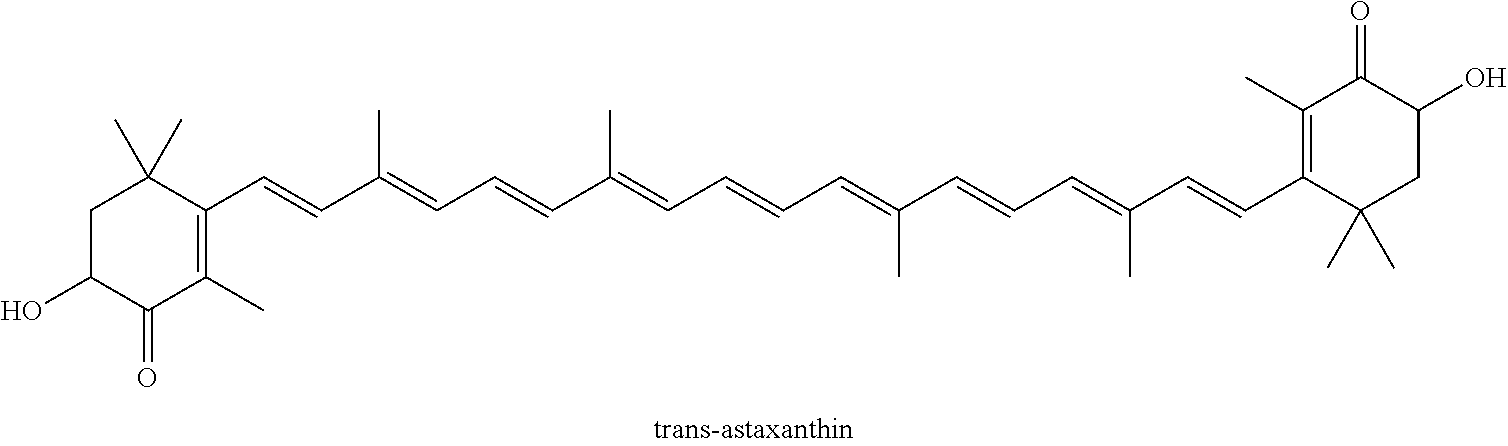
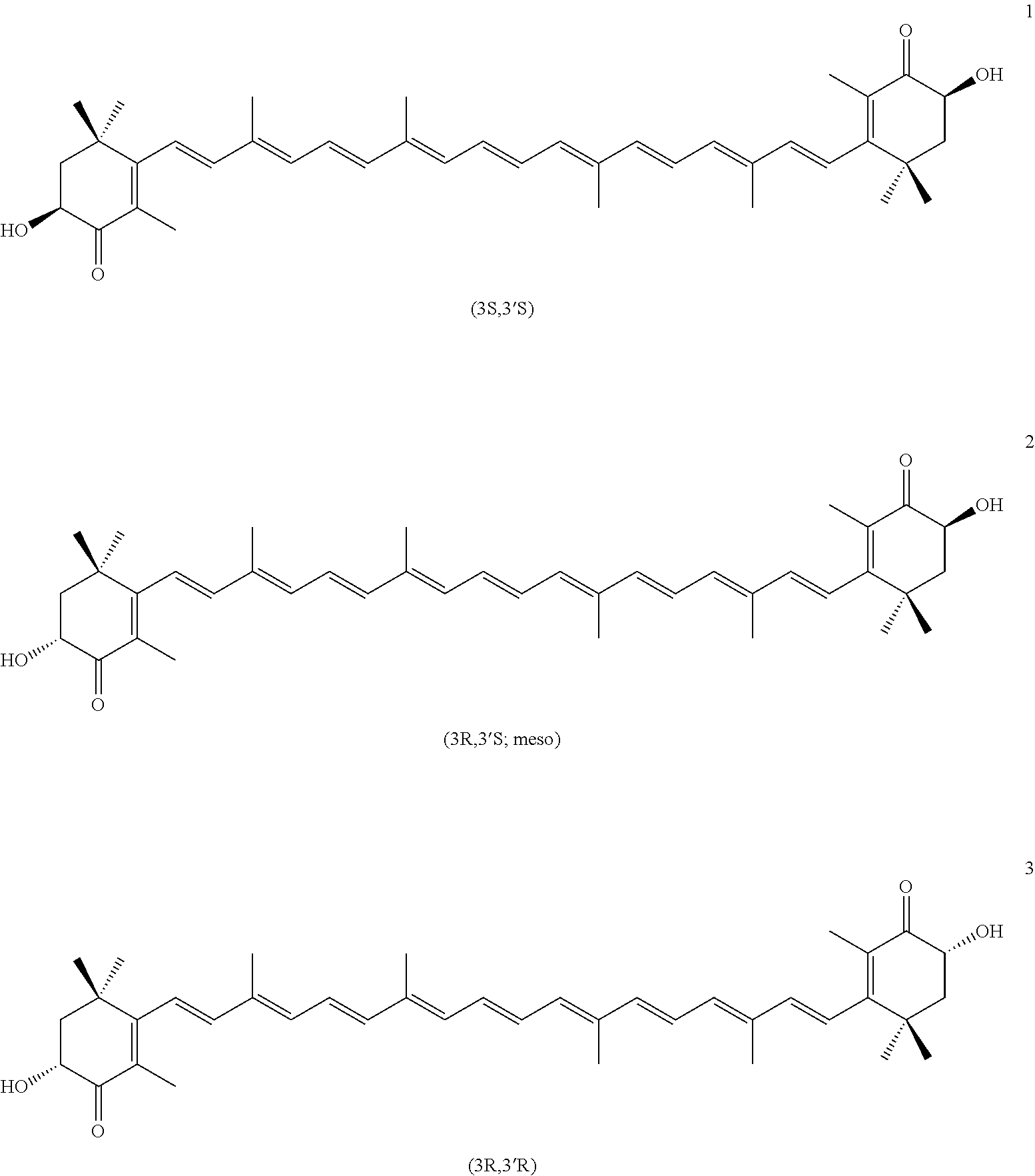
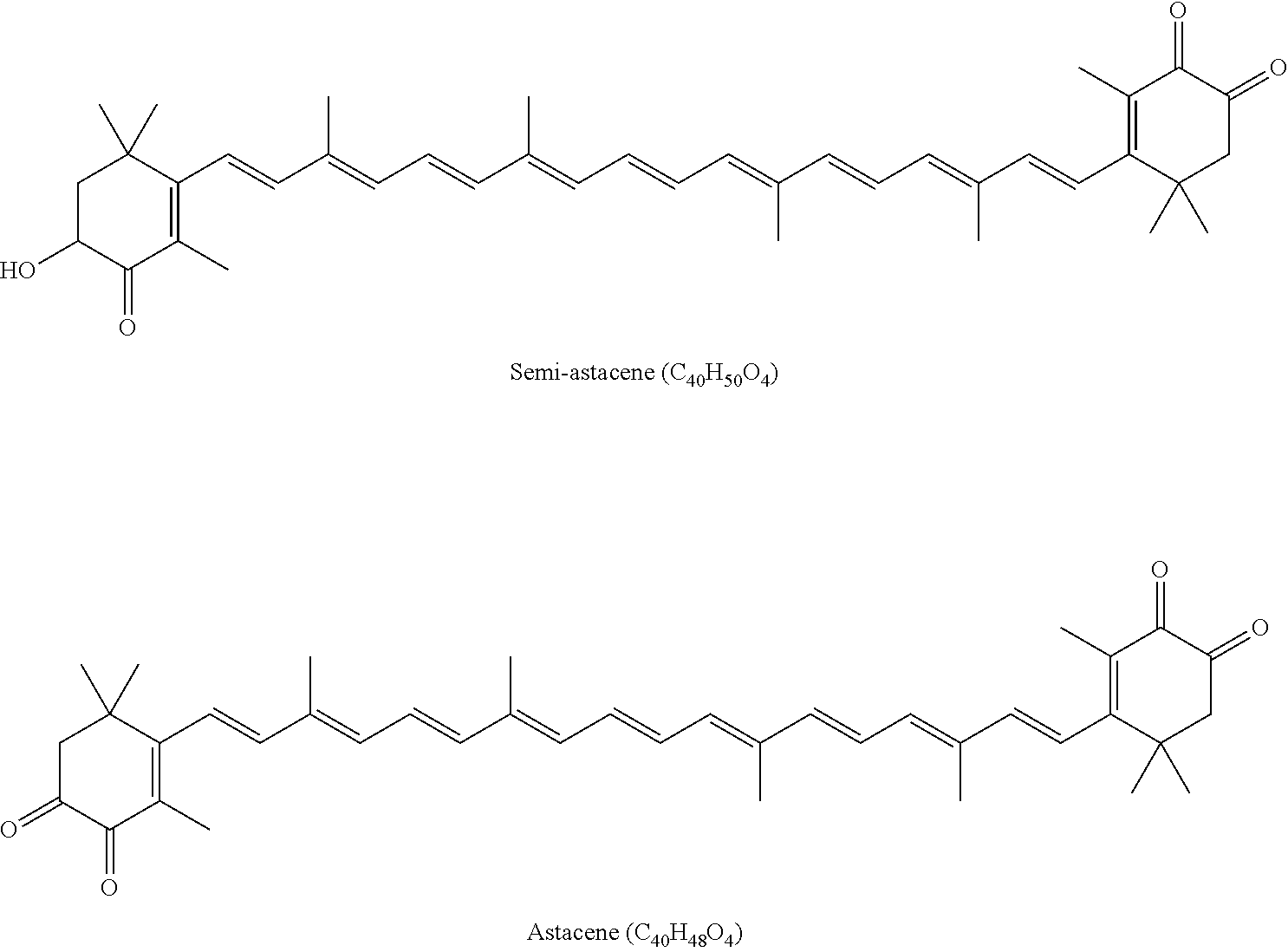
Carotenoid Compound Coming From Plant And Containing Natural Astaxanthin, Preparation Method Therefor, And Composition
a technology of astaxanthin and plant, which is applied in the field of astaxanthin compounds, can solve the problems of synthetic astaxanthin not being able to be converted into natural astaxanthin in animal bodies, the effect of biological absorption and pigmentation of synthetic astaxanthin is also worse than that of natural astaxanthin, and the purity of synthetic astaxanthin cannot be reduced. , to achieve the effect of increasing separation efficiency, reducing the purity of synthetic astaxan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0058]Fifty (50) g (UV content is 10.5 wt %) of Adonis amurensis extract (Adonis amurensis extract is obtained by extracting Adonis amurensis dried granules with a mixture of propane and butane and then is volatilized) is added to 2500 ml of absolute ethanol, heated to 75° C. under stirring, and kept for 1.0 hr to fully dissolve the Adonis amurensis extract. Next, 432 ml of NaOH solution with 45% of the mass concentration (w / w) is added dropwise to form a reaction mixture solution, and the alkali concentration of the reaction mixture solution is 2.0 mol / L after completion of the addition, and stirred at 75° C. for 4.0 hr. Next, 5000 ml of deionized water and 1000 ml of ethanol are added to it, and then a dark red crystal is precipitated after slowly stirring for 1.0 hr. After filtration, the resulting filter cake is washed with a mixture of ethanol and deionized water (the ratio of ethanol to deionized water is 2:1) twice, 600 ml of the mixture of ethanol and deionized water per tim...
example 2
[0063]100 g of Adonis amurensis extract (UV content is 10.5 wt %) is added to 3000 ml of glycerol, heated to 60° C. under stirring, and kept for 1.0 hr to fully dissolve the Adonis amurensis extract. 1000 ml of CH3ONa solution with the mass concentration of 25% (w / w) is added dropwise to form a reaction mixture solution, and the alkali concentration of the reaction mixture solution is 1.16 mol / L, and stirred at 60° C. for 0.5 hr. 6000 ml of deionized water and 1000 ml glycerol is added to it, and then a dark red crystal is precipitated after slowly stirring for 1.0 hr. After filtration, the filter cake is washed with a mixture of glycerol and deionized water (the ratio of glycerine and deionized water is 3:1) for twice, 500 ml of the mixture of glycerol and deionized water per time, to obtain a crystal, the crystal is dried in vacuum, to eventually obtain 4.8 g of a dark purple carotenoid crystal.
[0064]Its maximum absorption wavelength of the carotenoid crystal by UV detection is at...
example 3
[0066]100 kg adonis amurensis extract (UV content is 10.5 wt %) is added to 5000 L of propylene glycol, and is fully dispersed under stirring at 25° C. 123 L of KOH solution with a mass concentration of 25% (w / w) is added dropwise, and is stirred at 25° C. for 2.5 hr. After sampling and analysis with HPLC, it is found that the reaction is not complete, and the reaction is continued for 3.5 hr. After completion of the reaction monitored by HPLC, 5000 L of deionized water and 500 L of propylene glycol are added to it to terminate the reaction. After stirring slowly for 1.0 hr, a dark red crystal is precipitated. After filtration by pressure, a filter cake is washed with a mixture of propylene alcohol and deionized water (the ratio of propylene alcohol to water is 2:1) twice, 600 ml of the mixture of propylene alcohol and deionized water per time, to obtain a crystal, the crystal is dried in vacuum, to eventually obtain 6.8 Kg of a dark purple carotenoid crystal.
[0067]Its maximum absor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com