Silver nanowires, and production method and dispersion of the same
a silver nanowire and production method technology, applied in metal-working apparatuses, transportation and packaging, etc., can solve the problems of difficult in many cases to sinter silver nanowires, difficult to effectively remove polymer protective agents through washing operations, and difficult to substitute polymer protective agents with other surface protective agents, etc., to achieve the effect of increasing the probability of contact, reducing the amount of organic substances remaining on the surface of nanowires, and high adhesiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Silver Nanowires
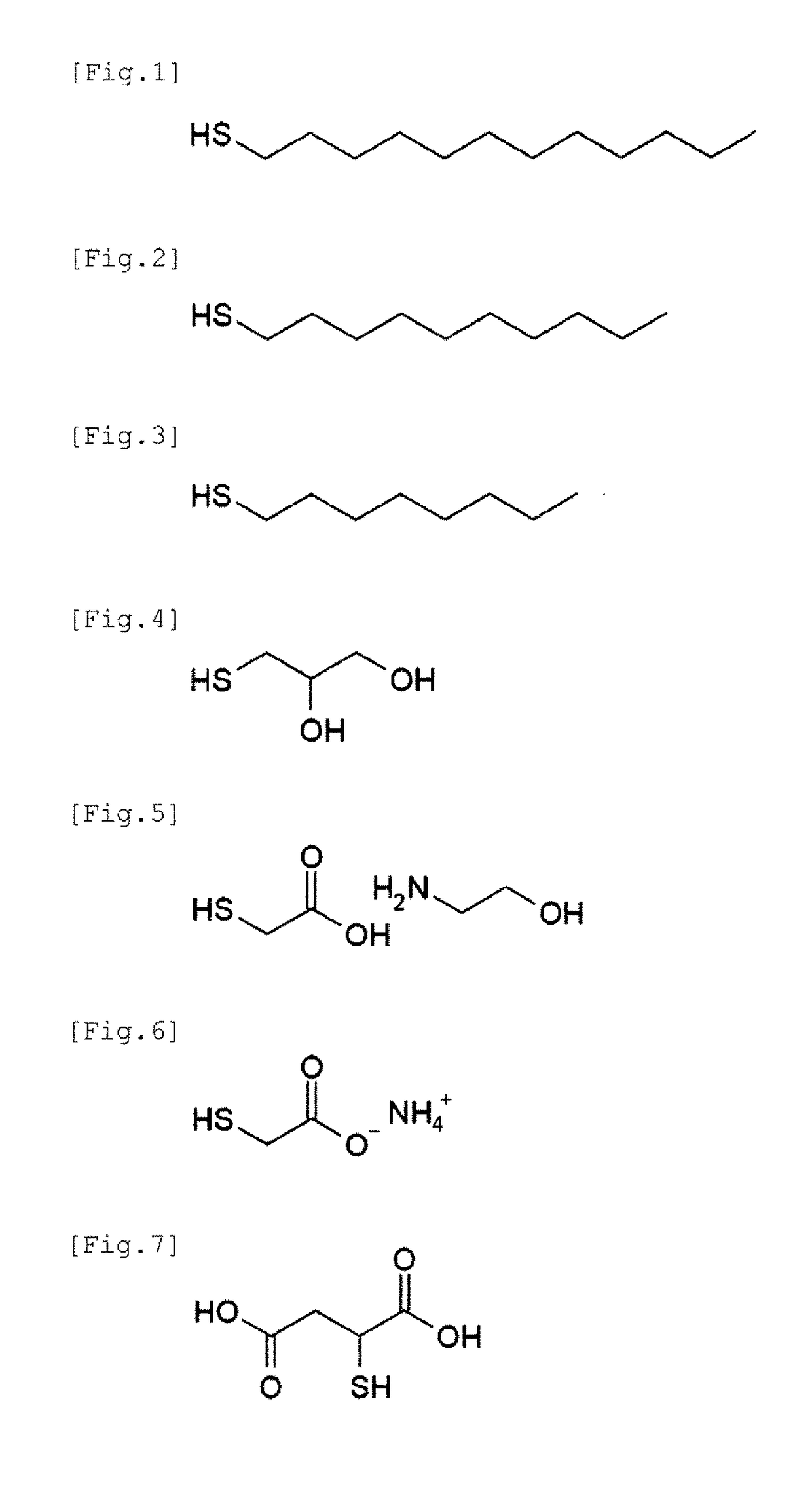
[0066]Polyvinylpyrrolidone (PVP) having a weight average molecular weight of 55,000 was provided as an organic protective agent.
[0067]At normal temperature, 2.5 g of PVP and 0.006 g (0.1 mmol) of sodium chloride were added to and dissolved in 60 g of ethylene glycol to prepare a solution X. In a container different from the above, 0.85 g (5.0 mmol) of silver nitrate was added to and dissolved in 7.65 g of ethylene glycol to prepare a solution Y.
[0068]Under an air atmosphere, the whole volume of the solution X was heated to 135° C. with stirring at 500 rpm, and then the solution Y was added at once into the solution X. After completing the addition of the solution Y, the stirring speed was changed to 100 rpm, and the solution was maintained at 135° C. for 3 hours while keeping the stirring state. Then, the reaction liquid was cooled to normal temperature.
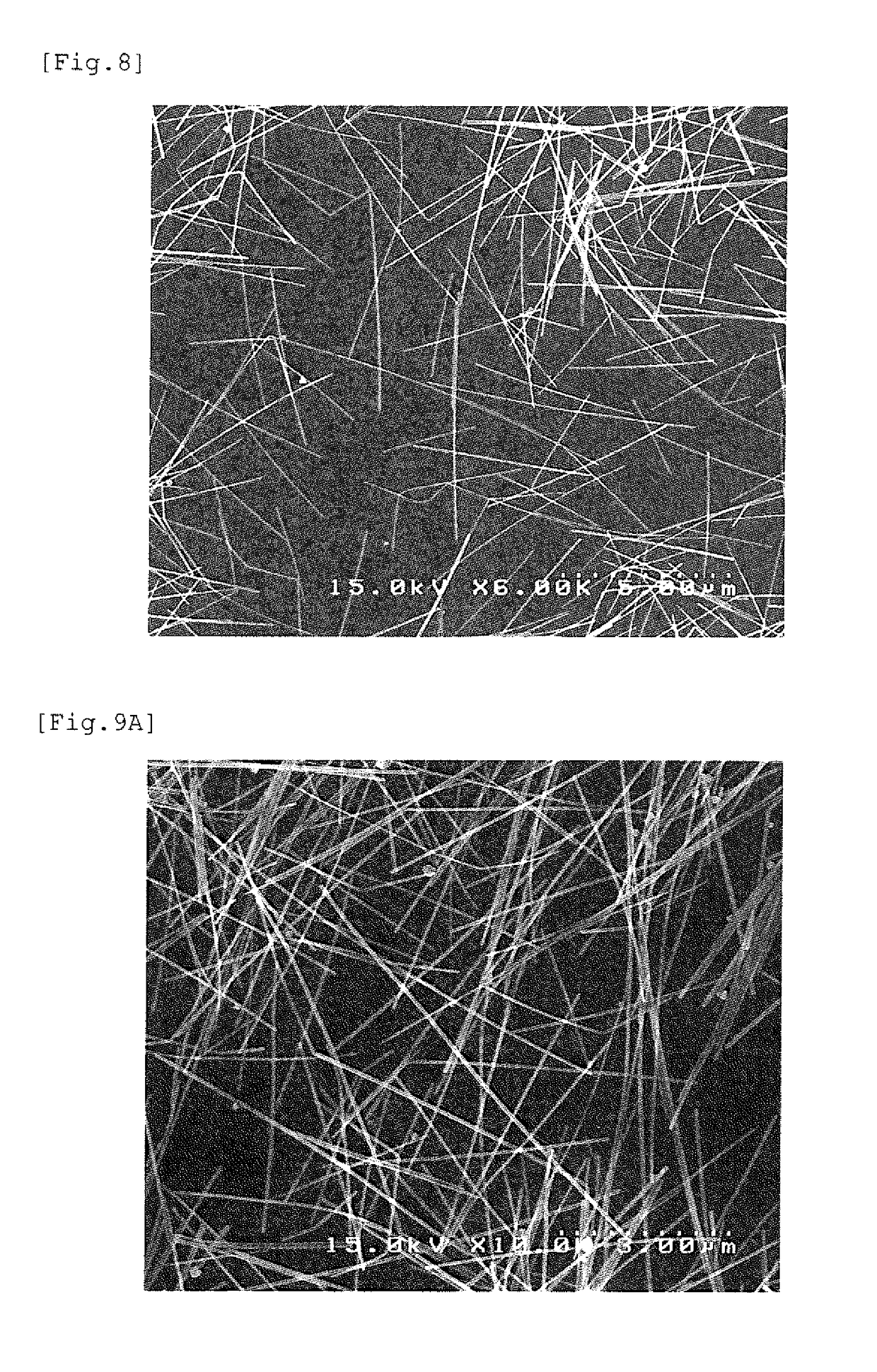
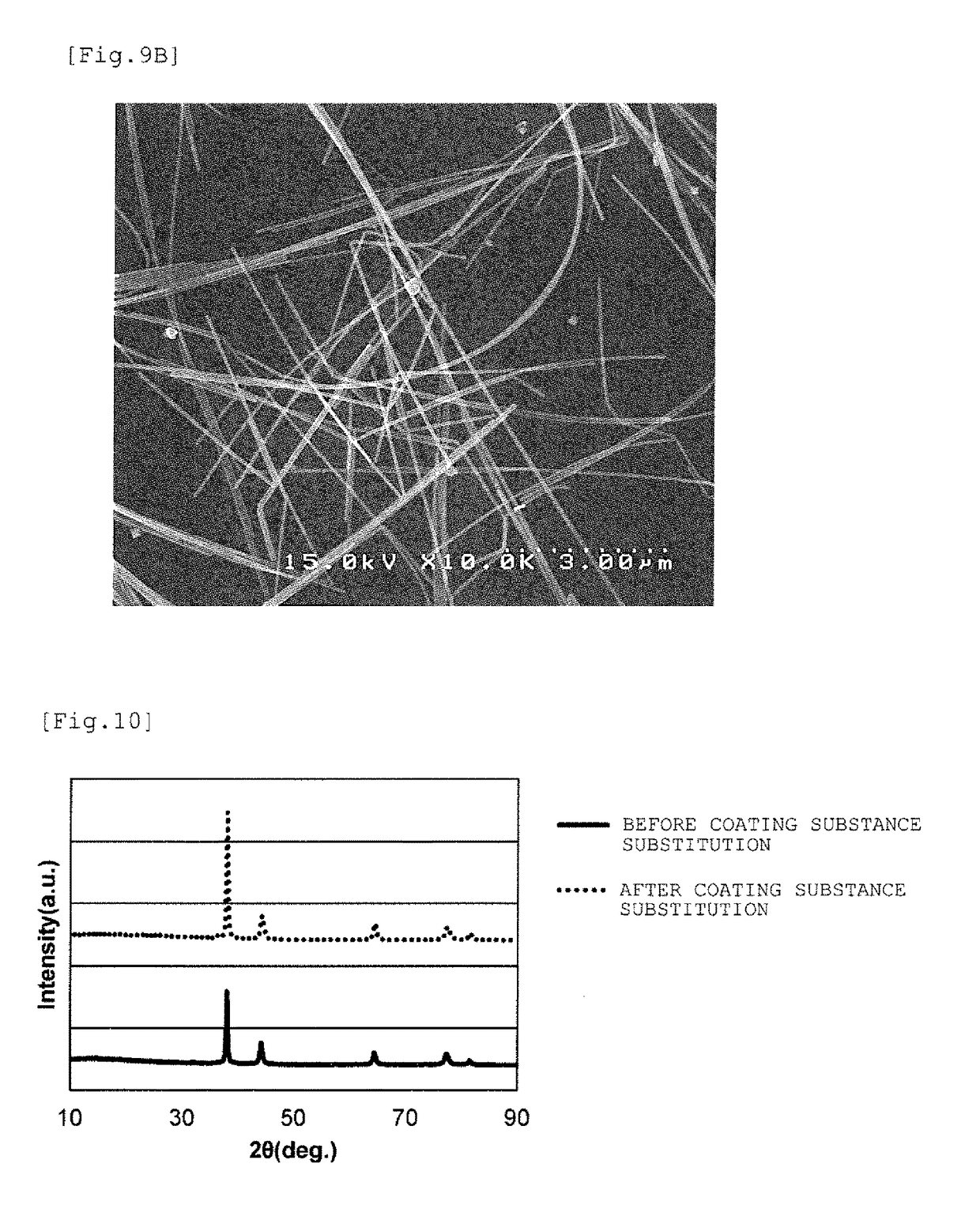
[0069]After cooling, washing was performed according to the following procedure to obtain a dispersion...
example 2
[0080]A nonpolar solvent dispersion of silver nanowires was obtained in the same manner as in Example 1 except that in the substitution operation of silver nanowire coating substance into a thiol, 1-decanethiol was used as the thiol for substitution on the wire surfaces. As the silver nanowires subjected to the substitution operation, the same nanowires as in Example 1 (PVP-coated silver nanowires) were used. According to a TG curve obtained under the same conditions as in Example 1, the ignition loss until 600° C. of the thiol (1-decanethiol)-coated silver nanowires after the coating substance substitution obtained in this example was 2.2%.
example 3
[0081]A nonpolar solvent dispersion of silver nanowires was obtained in the same manner as in Example 1 except that in the substitution operation of silver nanowire coating substance into a thiol, 1-octanethiol was used as the thiol for substitution on the wire surfaces. As the silver nanowires subjected to the substitution operation, the same nanowires as in Example 1 (PVP-coated silver nanowires) were used. According to a TG curve obtained under the same conditions as in Example 1, the ignition loss until 600° C. of the thiol (1-octanethiol)-coated silver nanowires after the coating substance substitution obtained in this example was 1.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com