Additive containing electrolytes for high energy rechargeable metal anode batteries
a rechargeable metal anode, additive technology, applied in the field of electrochemical cells, can solve the problems of poor coulombic efficiency and simply inability to use lithium salts in commercial rechargeable li-metal batteries, and achieve the effect of improving coulombic efficiency and electrolyte performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0089]We describe a general procedure for the preparation of novel electrolyte solutions suitable for commercially viable lithium metal batteries, the components of which are selected from embodiments herein. Both the concentration of the lithium salts and the relative ratios between the solvents or additives can be varied according to individual needs. The electrolyte solution is prepared in an environment that is moisture- and oxygen-free by dissolving one lithium salt or a combination of lithium salts selected from Table I in a mixture that is one part EC and two parts DMC by volume. The total concentration of lithium cations should occur in the range of 0.25 M to 5 M. A single additive or combination of additives is selected from Table II and dissolved in the electrolyte solution between 0 and 10 wt %. Dissolution occurs within 1-2 hours and the electrolyte may or may not be filtered prior to use.
[0090]Intercalation cathodes used in conjunction with the electrolyte according to ...
example 2
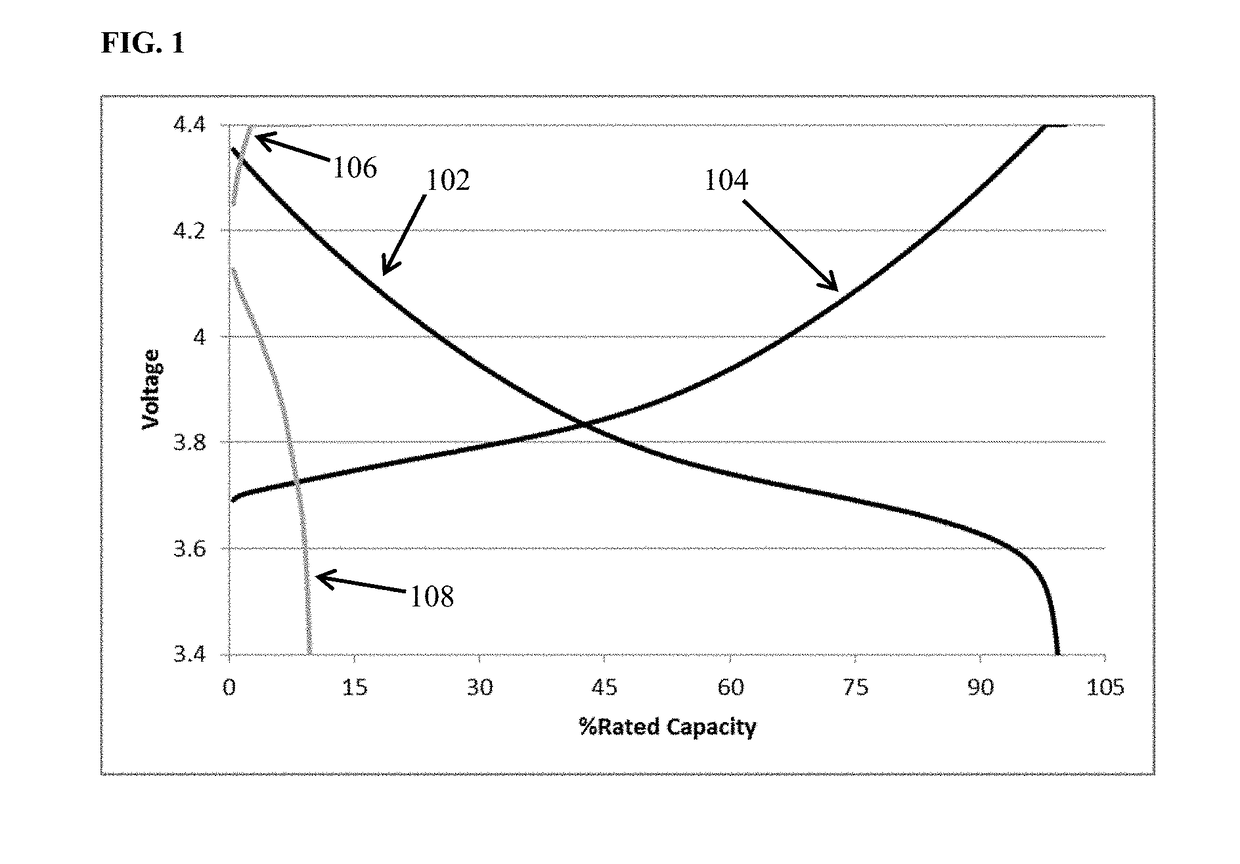
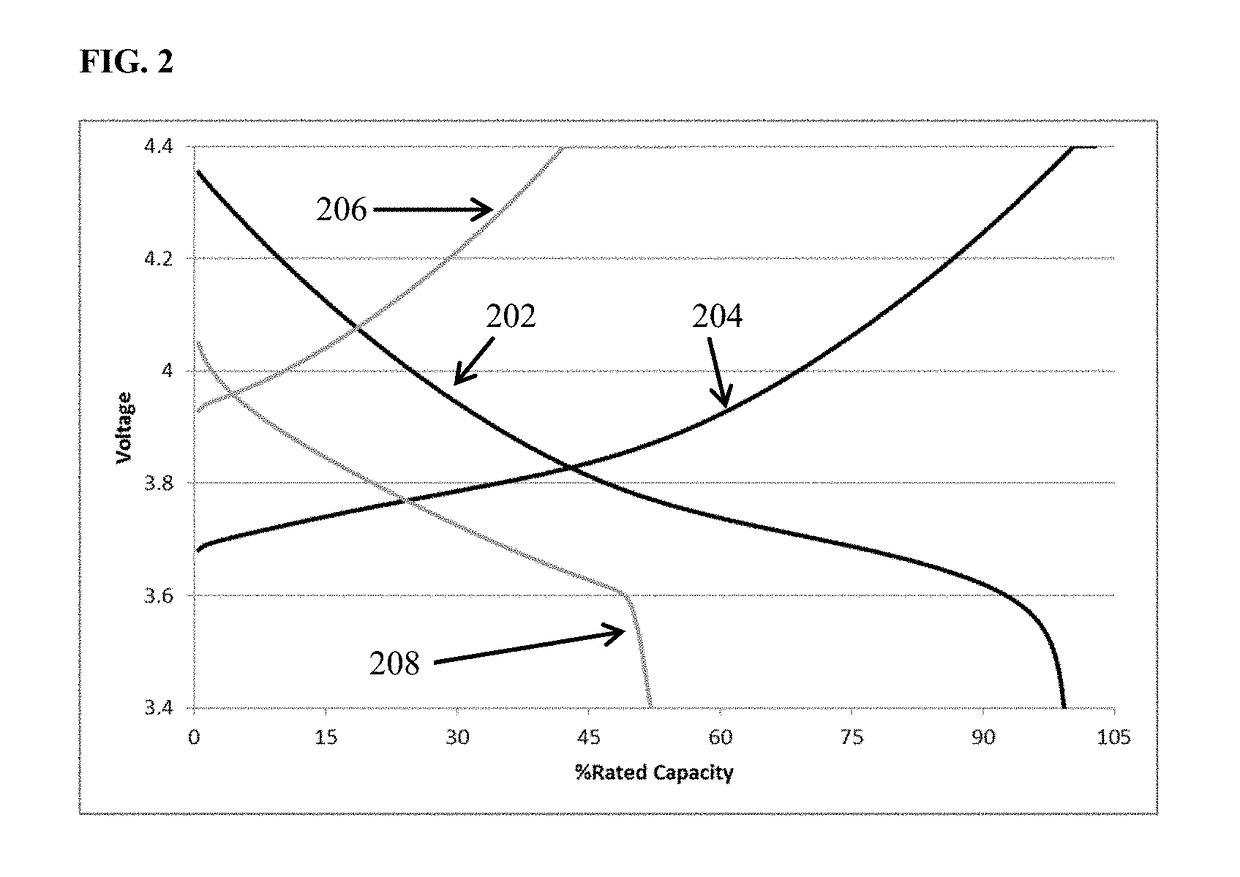
[0101]FIG. 1 depicts voltage vs. percent of rated capacity for cycle 1 (black) and cycle 60 (grey) of cells containing lithium transition metal oxide cathode, a lithium metal anode, and an 0.85 M lithium bis(oxalato)borate in EC:DMC 1:2 (v / v) electrolyte. This is a zero excess lithium cell (i.e., N / P<1, all the lithium in the as built cell resides in the cathode.) The cell cycled at room temperature utilizing constant current discharge at one C-rate to 100% depth of discharge. It is apparent from the figure that the capacity retention at cycle 60 is only about 10% of the value achieved upon the initial discharge. FIG. 2 depicts voltage vs. percent of rated capacity for a comparative cell containing electrolyte additive among those disclosed herein. Cycle 1 (black) and cycle 60 (grey) of cells containing lithium transition metal oxide cathode, a lithium metal anode, and an 0.85 M lithium bis(oxalato)borate with an additive comprising 1 wt % dodecyl isocyanate dissolved in EC:DMC 1:2 ...
example 3
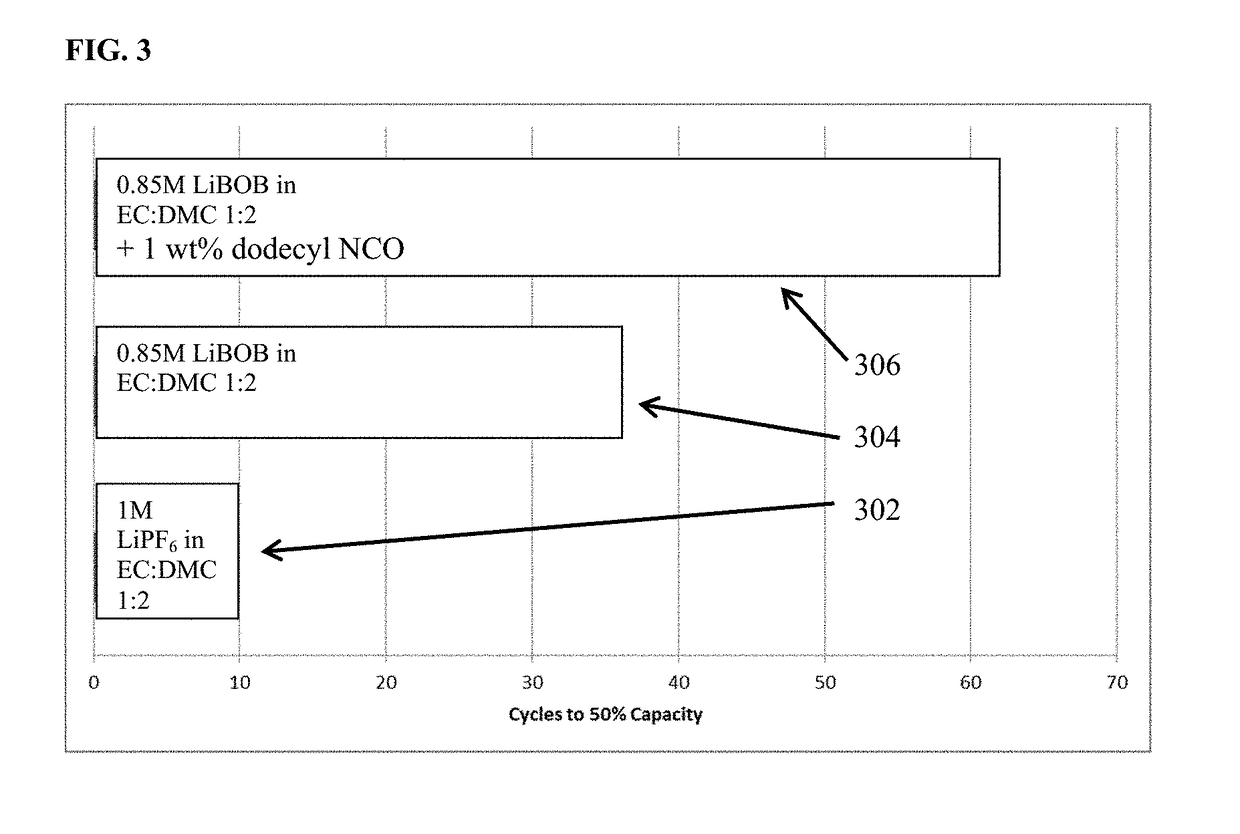
[0102]Three cells comprised of a lithium transition metal oxide cathode, a lithium metal anode, an electrolyte and an porous, electronically resistive separator were cycled at room temperature utilizing constant current discharge at one C-rate to 100% depth of discharge. FIG. 3 depicts the number of charge-discharge cycles to 50% rated capacity for each of the three cells. The electrolyte in one cell comprises 1 M LiPF6 in EC:DMC 1:2 (v / v) while the electrolyte in the second cell comprises 0.85 M LiBOB in EC:DMC 1:2 (v / v), and the third cell comprises 0.85 M LiBOB and an additive 1 wt % dodecyl isocyanate in EC:DMC 1:2 (v / v). The 1M LiPF6 in EC:DMC 1:2 (v / v) requires only 10 cycles to fade down to 50% capacity while the electrolyte comprised of 0.85 M LiBOB in EC:DMC 1:2 (v / v) enables 50% capacity retention at cycle 36, and additive containing electrolyte, namely 0.85 M LiBOB and an additive 1 wt % dodecyl isocyanate in EC:DMC 1:2 (v / v) reaches the 50% capacity retention mark at 62 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Coulombic efficiency | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com