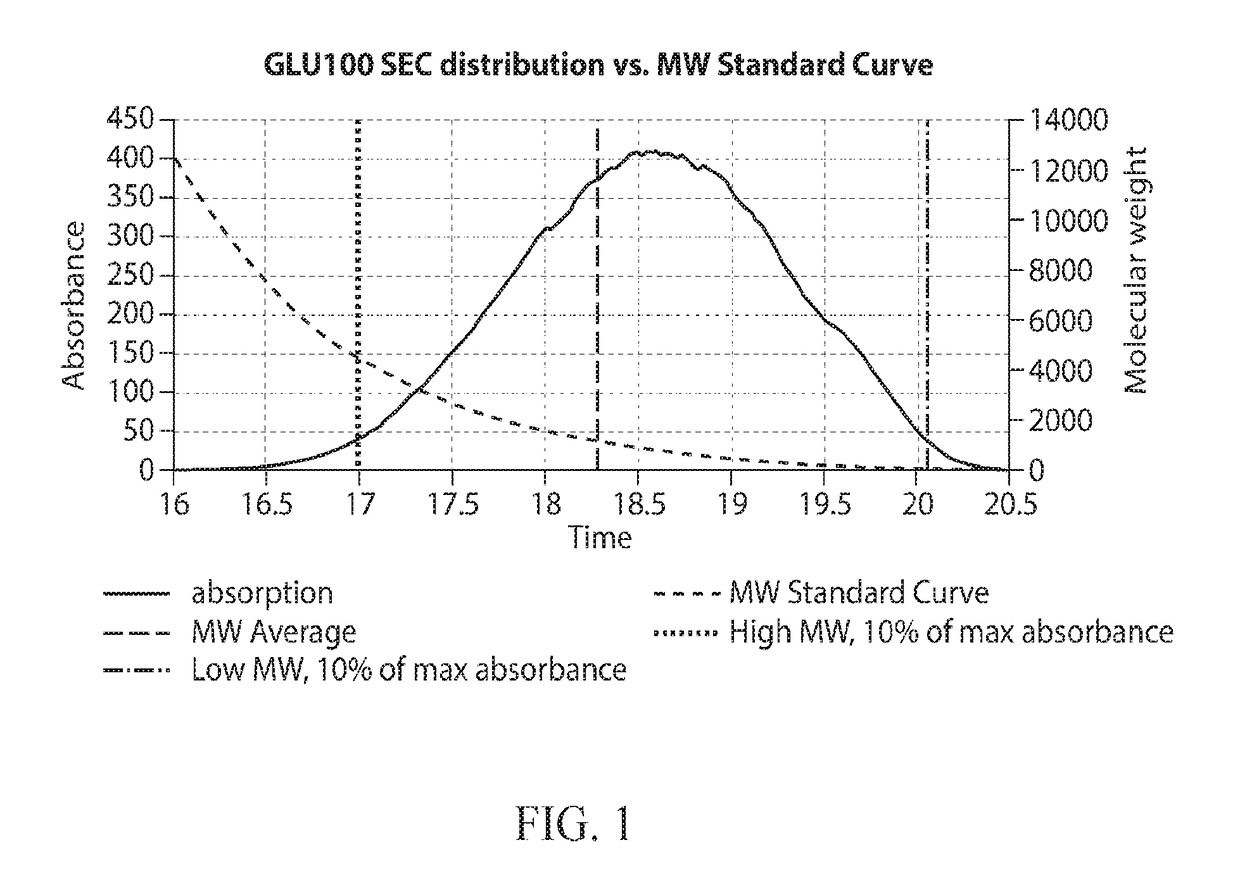
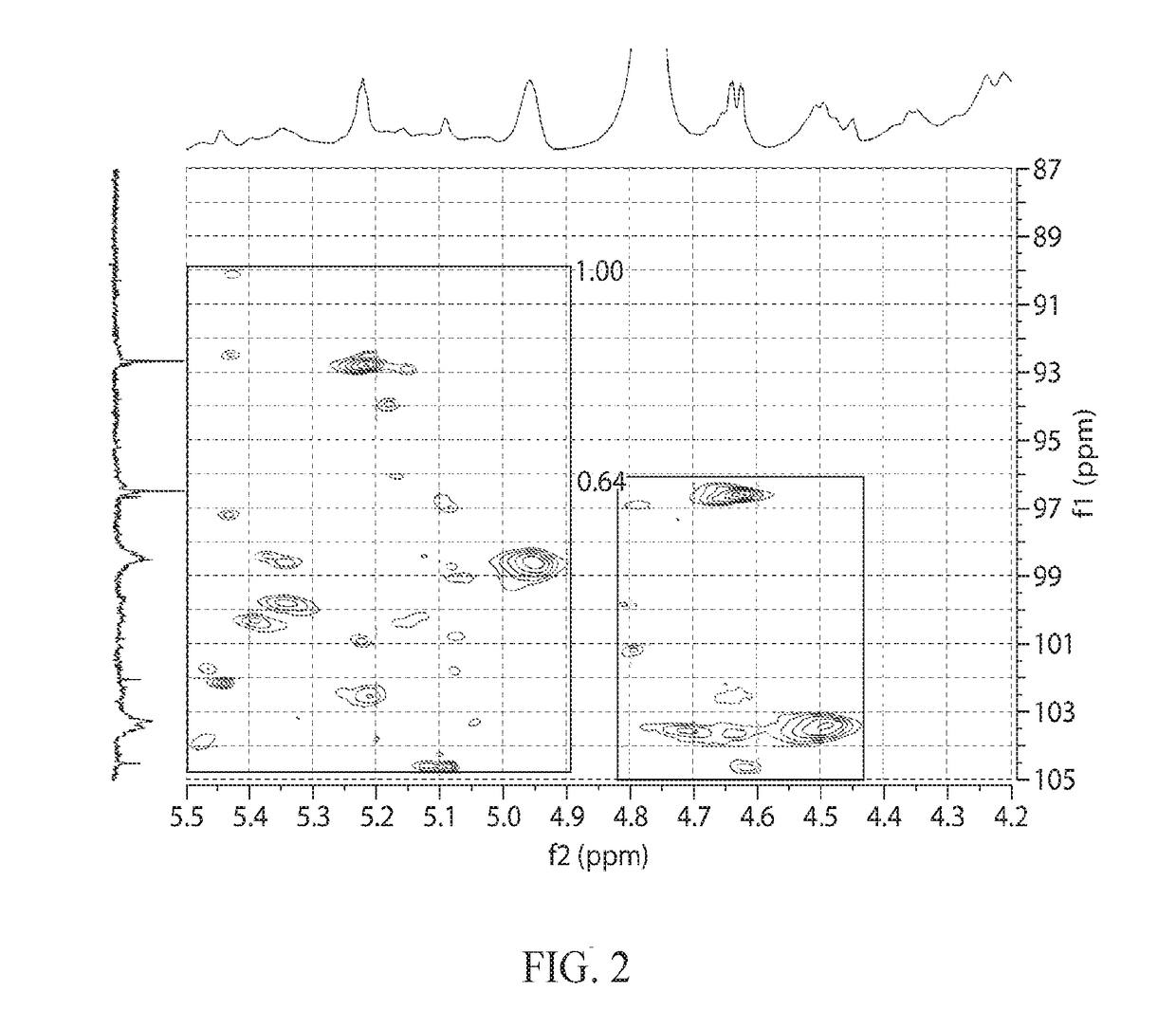
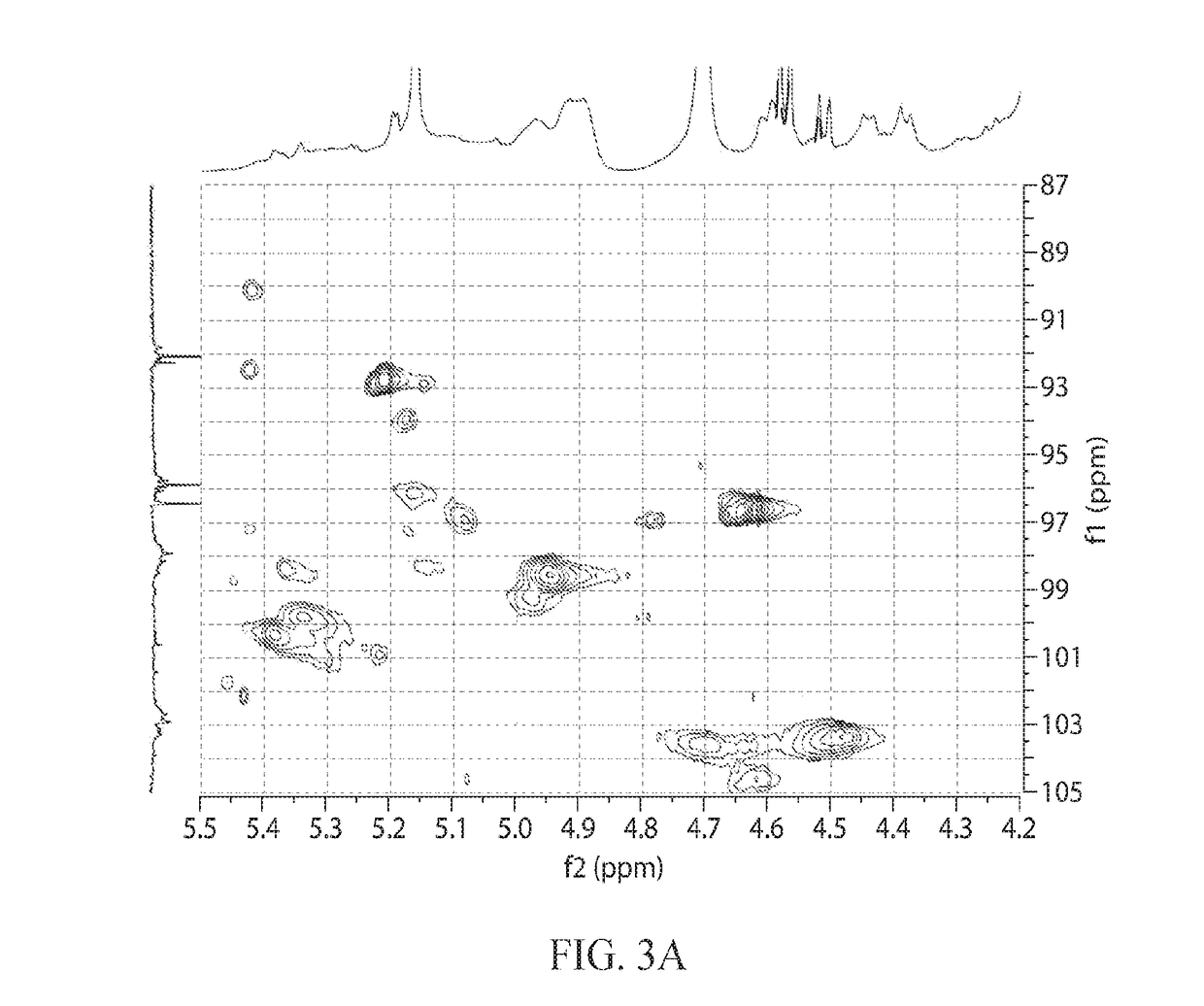
Glycan therapeutic compositions and related methods thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Glycan Therapeutics
[0484]To a round bottom flask equipped with an overhead stirrer and a jacketed short-path condenser was added one or more mono- or disaccharides along with 3-20% by dry weight of one or more of the catalysts described in U.S. Pat. No. 8,466,242 and WO 2014 / 031956, which are incorporated herein by reference in their entirety. Water or another compatible solvent (1.54 equiv) was added to the dry mixture and the slurry was combined at approximately 100 rpm using a paddle sized to match the contours of the selected round bottom flask as closely as possible. The mixture was then heated to 80-155° C. Once the solids achieved a molten state, the vessel was placed under 10-1000 mbar vacuum pressure. The reaction was stirred for 30 minutes to 8 hours, constantly removing water from the reaction. Reaction progress was monitored by HPLC. When sufficient oligomerization had occurred, the stirrer was shut off, the reaction was cooled to room temperature and vented to atm...
example 2
[0491]Oligosaccharides synthesized as in Example 1 were dissolved in deionized water to a final concentration of 25-50 Brix. The material was then exposed to at least 2 mass equivalents of Dowex Monosphere 88 ion exchange resin. Exposure may occur by swirling in a flask at 120-170 rpm or by filtration through a wet slurry packed column as long as the residence time is sufficient for the solution to achieve a final pH between 3 and 5. The oligomer solution was isolated by filtration (as in the case of swirled reactions) or elution (as in the case of column filtration) and the process was repeated with Dowex Monosphere 77 ion exchange resin in an analogous fashion until the solution pH was above 5.5. Finally the solution was exposed to Dowex Optipore SD-2 Adsorbent decolorizing resin until the solution was sufficiently clarified and filtered through a 0.2 micron filter to remove residual resin and resin fines. The final solution was then concentrated to 50-85...
example 3
ion of Glycan Therapeutics by Removal of Low Molecular Weight Species
[0493]Oligomers prepared and purified as in Examples 1 and 2 were modified so as to remove low molecular weight species. The separation was achieved by osmotic separation. Approximately 45 cm of 1.0 kD MWCO Biotech CE dialysis tubing (31 mm flat width) from Spectrum Labs was placed into deionized water and soaked for 10 minutes, then one end was sealed with a dialysis tubing clip. A 25 Brix solution of 8 grams dry oligosaccharide was sterile filtered and sealed into the tube with a second clip along with a few mL of air to permit the tube to float. The filled tube was then placed in a 3 gallon tank of deionized water which was stirred with sufficient force to induce slow swirling of the sealed tubes. After 8 hours, the water in the tank was replaced and the tube was allowed to stir for an additional 16 hours. Once the dialysis was complete and the material had a DP2+ yield greater than 95% and a DP3+ yield greater ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com