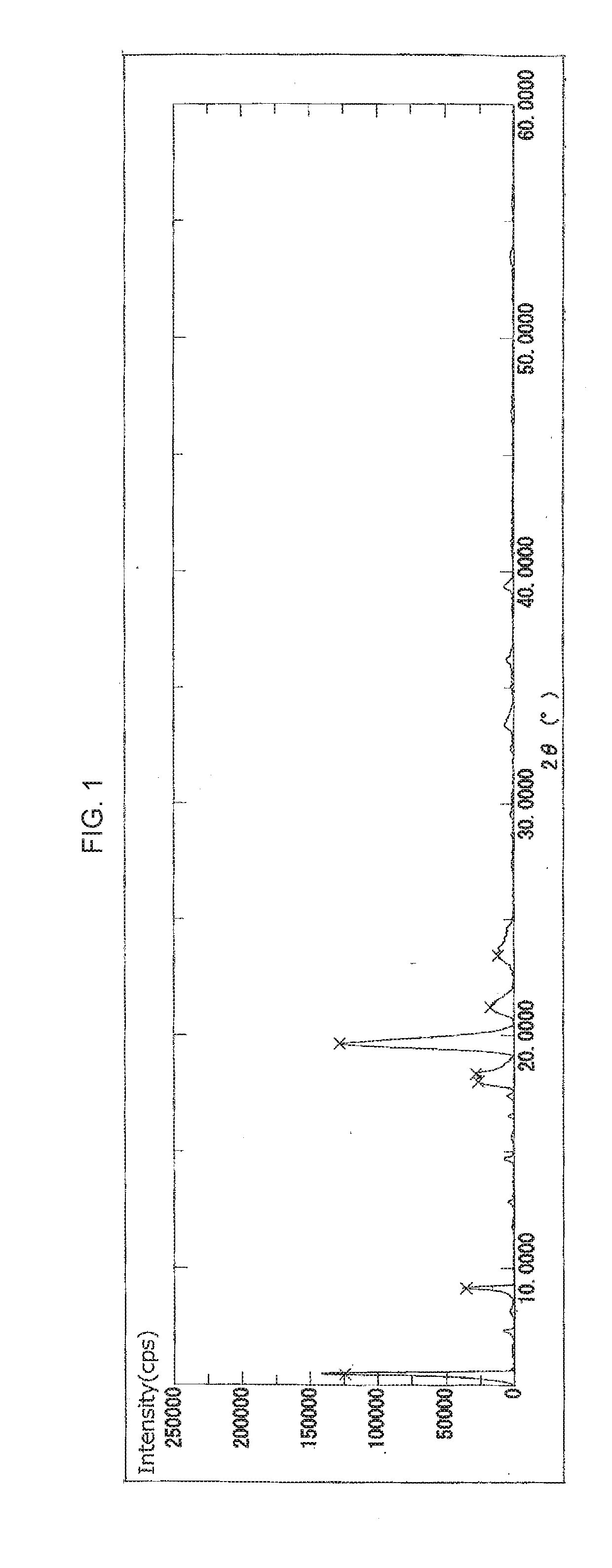
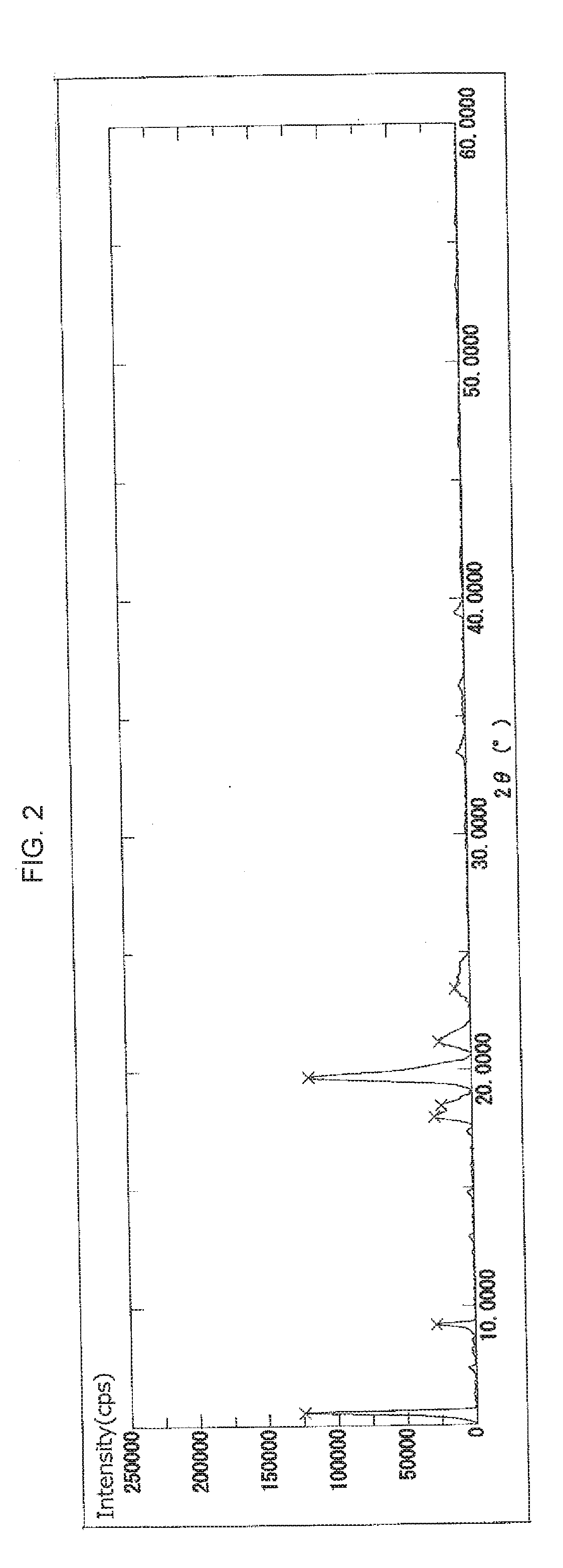
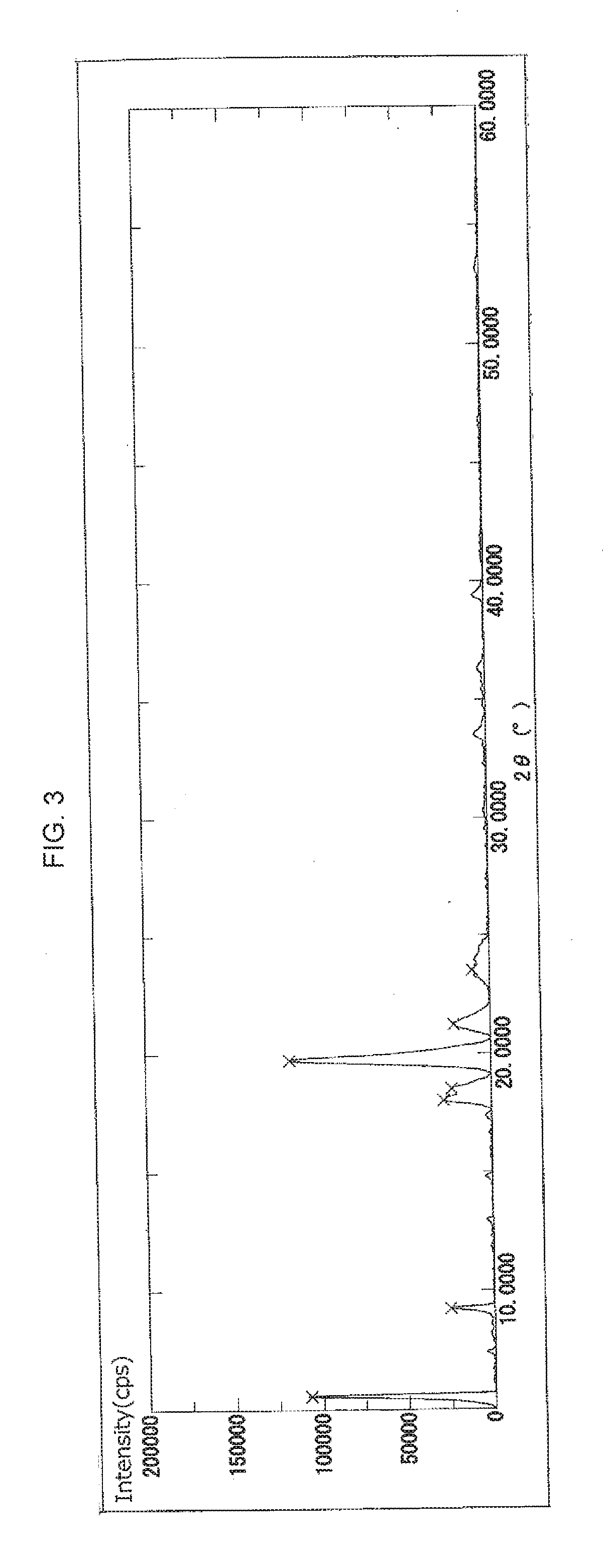
Crystal of cyclic phosphonic acid sodium salt and method for manufacturing same
a technology sodium salt, which is applied in the field of crystallization of cyclic phosphonic acid sodium salt, can solve the problems of strong acidity of the reaction system, inability to synthesis on a large scale, and product prone to decomposition, and achieves excellent storage stability, high yield, and simple manner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0255]The present invention is described in further detail with reference to Synthesis Examples, Examples, and Comparative Examples. However, the present invention is not limited to the following Examples.
Step (A)
Synthesis Example A1: (Step A: R2=n-Propyl)
Synthesis of 2,2-di-n-propyl-5-(hydroxymethyl)-1,3-dioxane (3b)
[0256]
[0257]2-Hydroxymethyl-1,3-propanediol (2) (3.0 g) was dissolved in 30 mL of tetrahydrofuran, and 4.74 mL of 4-heptanone and 53.8 mg of p-toluenesulfonic acid monohydrate were added thereto, followed by heating under reflux for 3.5 hours using a Dean-Stark trap. During the reaction, distilled tetrahydrofuran was discarded, and new tetrahydrofuran was added to the reaction mixture. Thereafter, 0.39 mL of triethylamine was added to the reaction mixture to stop the reaction, and tetrahydrofuran was distilled off under reduced pressure. Then, 30 mL of ethyl acetate and 30 mL of water were added to the residue, and the layers were separated. After the organic layer was ...
synthesis example c2-1 (
Step C: Solvent=Acetone+Methyl Isobutyl Ketone)
Synthesis of 2,2-dimethyl-5-iodomethyl-1,3-dioxane (5a)
[0289]
[0290]The compound (4a) obtained in Synthesis Example B1 (300 mg) was dissolved in 3 mL of acetone and 3 mL of methyl isobutyl ketone in an argon atmosphere, and 9.3 μL of triethylamine and 301 mg of sodium iodide were further added thereto, followed by heating under reflux for 2 hours. Acetone and methyl isobutyl ketone in the reaction solution were distilled off under reduced pressure, 6 mL of CH2Cl2 and 6 mL of water were added thereto, and the layers were separated. The water layer was extracted twice with 6 mL of CH2Cl2, the organic layers were washed with 3 mL of 5% sodium thiosulfate and 3 mL of 1% sodium hydrogen carbonate water. After the organic layers were further washed with 6 mL of saturated brine and dried over magnesium sulfate, CH2Cl2 was distilled off under reduced pressure. The resulting residue was purified with silica gel chromatography (n-hexane:ethyl acet...
synthesis example c2-2 (
Synthesis of 2,2-dimethyl-5-iodomethyl-1,3-dioxane (5a)
[0291]
[0292]The compound (4a) obtained in Synthesis Example B1 (300 mg) was dissolved in 6 mL of acetone in an argon atmosphere. Then, 9.3 μL of triethylamine and 301 mg of sodium iodide were further added thereto, and the mixture was heated under reflux for 2 hours. Acetone in the reaction solution was distilled off under reduced pressure, 6 mL of CH2Cl2 and 6 mL of water were added thereto, and the layers were separated. The water layer was extracted twice with 6 mL of CH2Cl2, and the organic layers were washed with 3 mL of 5% sodium thiosulfate and 3 mL of 1% sodium hydrogen carbonate water. After the organic layers were further washed with 6 mL of saturated brine and dried over magnesium sulfate, CH2Cl2 was distilled off under reduced pressure. The resulting residue was purified with silica gel chromatography (n-hexane:ethyl acetate=10:1) to obtain 204 mg of compound (5a) (yield: 59%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com