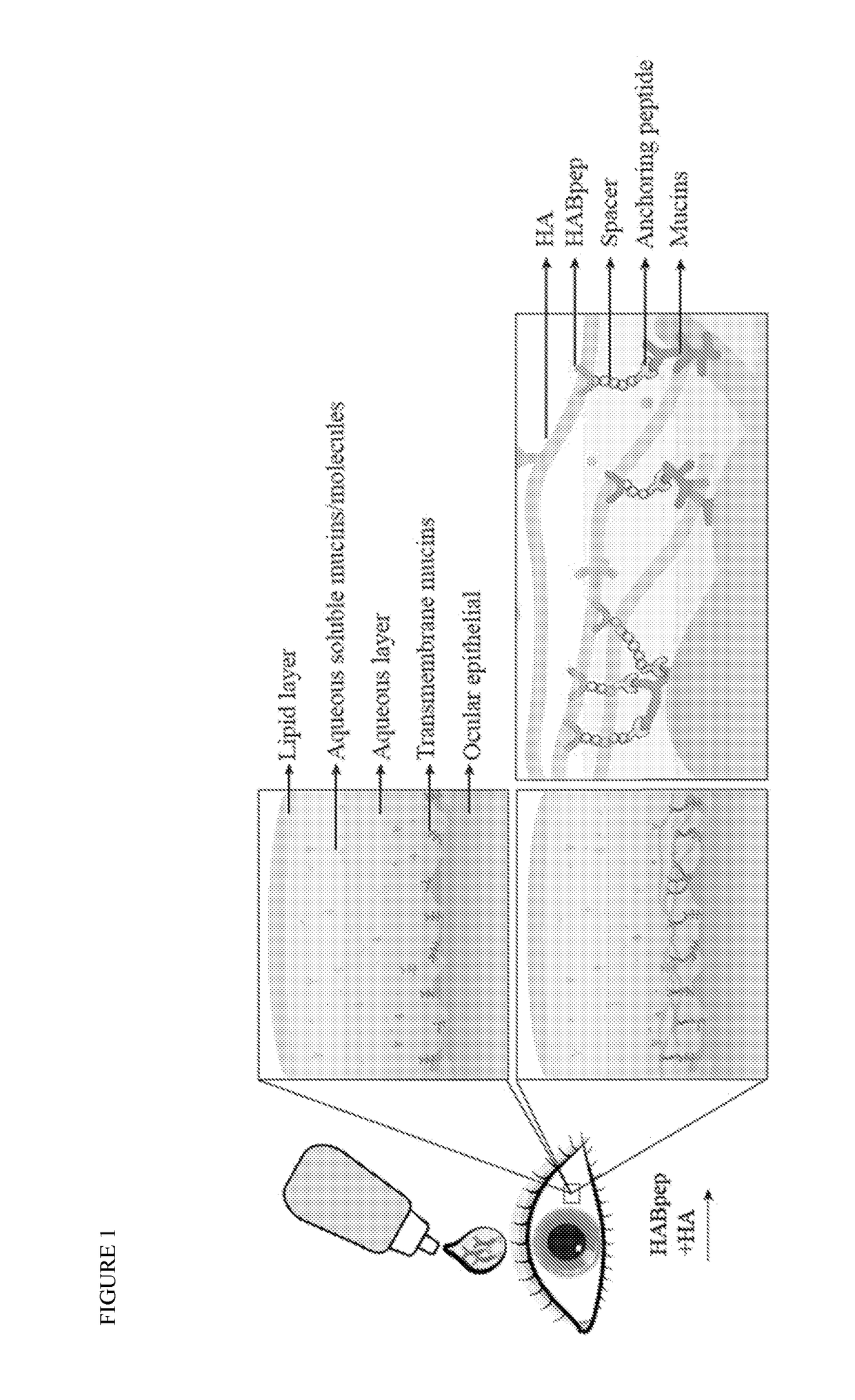
An eye drop solution for enhanced hyaluronic acid retention and delivery
a technology of hyaluronic acid and eye drops, which is applied in the direction of drug compositions, peptide/protein ingredients, sense disorders, etc., can solve the problems of low ocular residence time (10 min), affecting the performance of everyday activities, and high concentration of ha-based eye drops, etc., to achieve the effect of enhancing the availability of ha
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0141]Peptide screening: selecting peptide that binds to the ocular tissues.
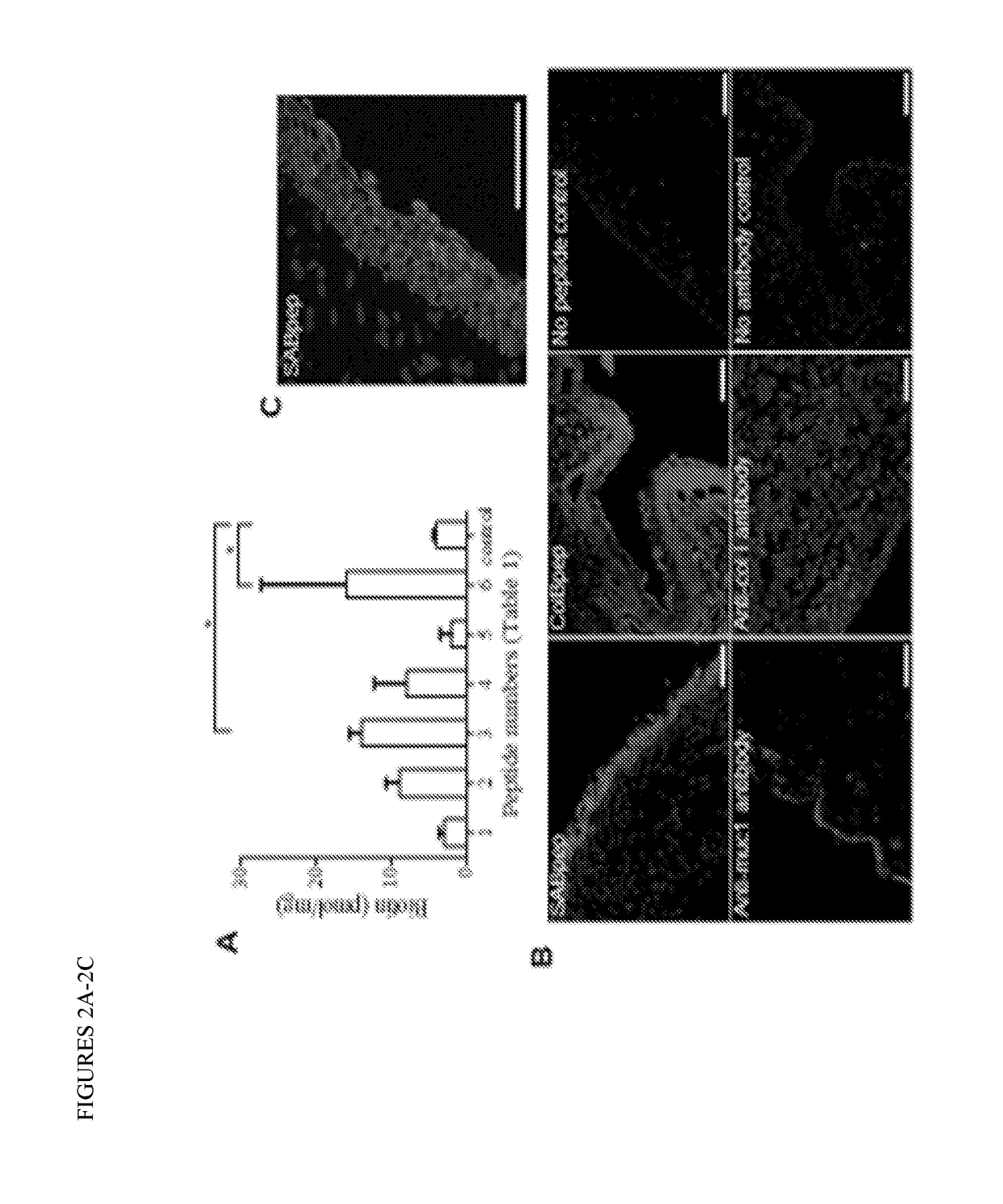
[0142]We screened multiple peptides that are supposed to bind different sites of the ocular tissues and cells (data not shown). Out of the 6 screened peptides, a sialic acid binding peptide GGSPYGRC (SEQ ID NO: 15) hereafter respectively referred to as SABpep, and a collagen binding peptide SYIRIADTNIT (SEQ ID NO: 12) hereafter respectively referred to as ColBpep were chosen for further experiments (FIG. 2A). The chosen SABpep and ColBpep exhibited little to no cytotoxicity to epithelial cells as well (data not shown)
example 2
[0143]SABpep and ColBpep binding on conjunctiva tissue.
[0144]Rabbit conjunctival sections bound SABpep specifically on the epithelial lining of the conjunctiva (FIG. 2B), indicating the strong presence of sialic acid on mucin-1 as compared to mucin-1 antibody staining. A higher magnification confirmed that SABpep staining was localized to only on cell membranes (FIG. 2C). Conversely, as expected, ColBpep staining was localized on conjunctiva tissue more indiscriminately, which is similar to type I collagen antibody staining (FIG. 2B). The binding specificity of SABpep was further proven by sialidase digestion and pre-incubation with sialic acid. SABpep could not bind to conjunctival epithelium if the section was digested by sialiadase before staining. However, SABpep still stained the epithelium even after preincubation with sialic acid solution (data not shown).
example 3
[0145]Mucin and HA binding studies by Quartz Crystal Microbalance with Dissipation (QCM-D) monitoring.
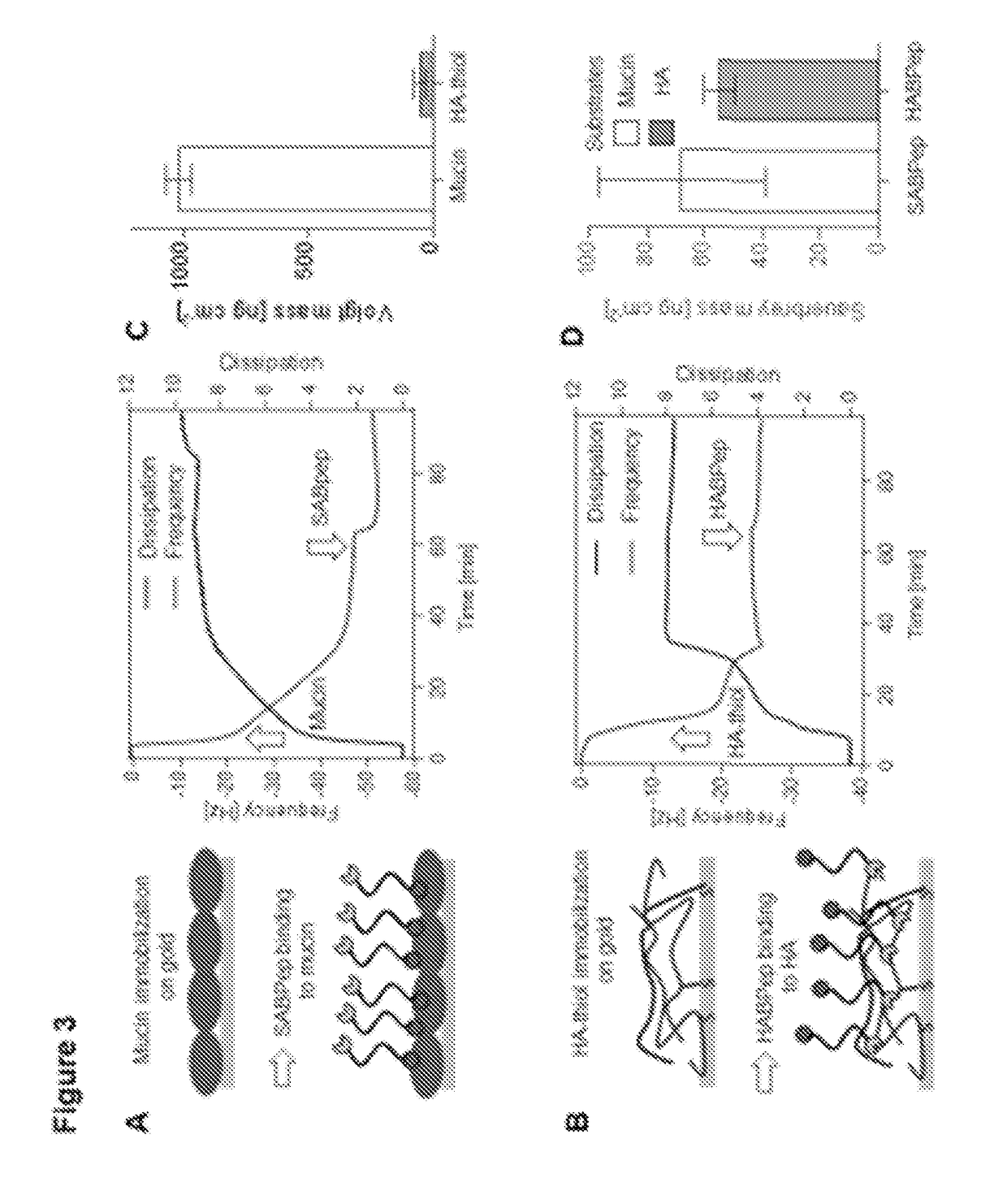
[0146]Surface binding of polymer-peptide conjugates to mucin and HA was directly measured using QCM-D. First, sialic acid bearing mucin was adsorbed on gold substrates to mimic the interface at the corneal epithelium (FIG. 3A), as native mucin from bovine submaxillary glands is abundant with O-linked oligosaccharide chains, and can be used as a source of sialic acids. A visco-elastic layer of highly hydrated mucin was formed as shown by a frequency change of Δf=−32 Hz and dissipation change of ΔD=7. Then SABpep-PEG-HABpep was applied to the mucin layer and specific binding was indicated with a frequency change of Δf=−5 Hz. In turn, SABpep-PEG-HABpep was immobilized onto HA substrates with a Δf=−3 Hz (FIG. 3B). The Voigt mass quantified the consistent formation of visco-elastic substrates Mucin and HA (FIG. 3C). In turn, HApep binding showed a frequency but not a dissipation change. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com