Ceritinib formulation
a technology of ceritinib and formulation, which is applied in the field of ceritinib formulation, can solve the problems of affecting the compressibility of tablets, difficult formulation of ceritinib, and general adverse effects of drug load, so as to reduce or alleviate at least one symptom, increase progression-free survival, and overall survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0098]The following Examples illustrate the disclosure described above; they are not, however, intended to limit the scope of the disclosure in any way. The beneficial effects of the ceritinib for use in the treatment according to the present disclosure, or methods as disclosed herein can also be determined by other test models known as such to the person skilled in the pertinent art.
[0099]All experiments described hereinafter were performed by using the following laboratory or manufacturing equipment:
Granulator: Top or bottom driven High shear mixer.
Sieve: Manual hand sieve, oscillating and rotating sieve mill
Drying: Fluidized bed dryers
Tableting machine: Compaction simulator / Excentric tableting machine, rotary tableting machine
Roller compactor: Cantilevered roll design and feature a vertical tapered deed screw system
Film coating drum: Perforated drum coater
Dissolution apparatus: USP II Dissolution apparatus
examples 1 to 3a
ulation Technology
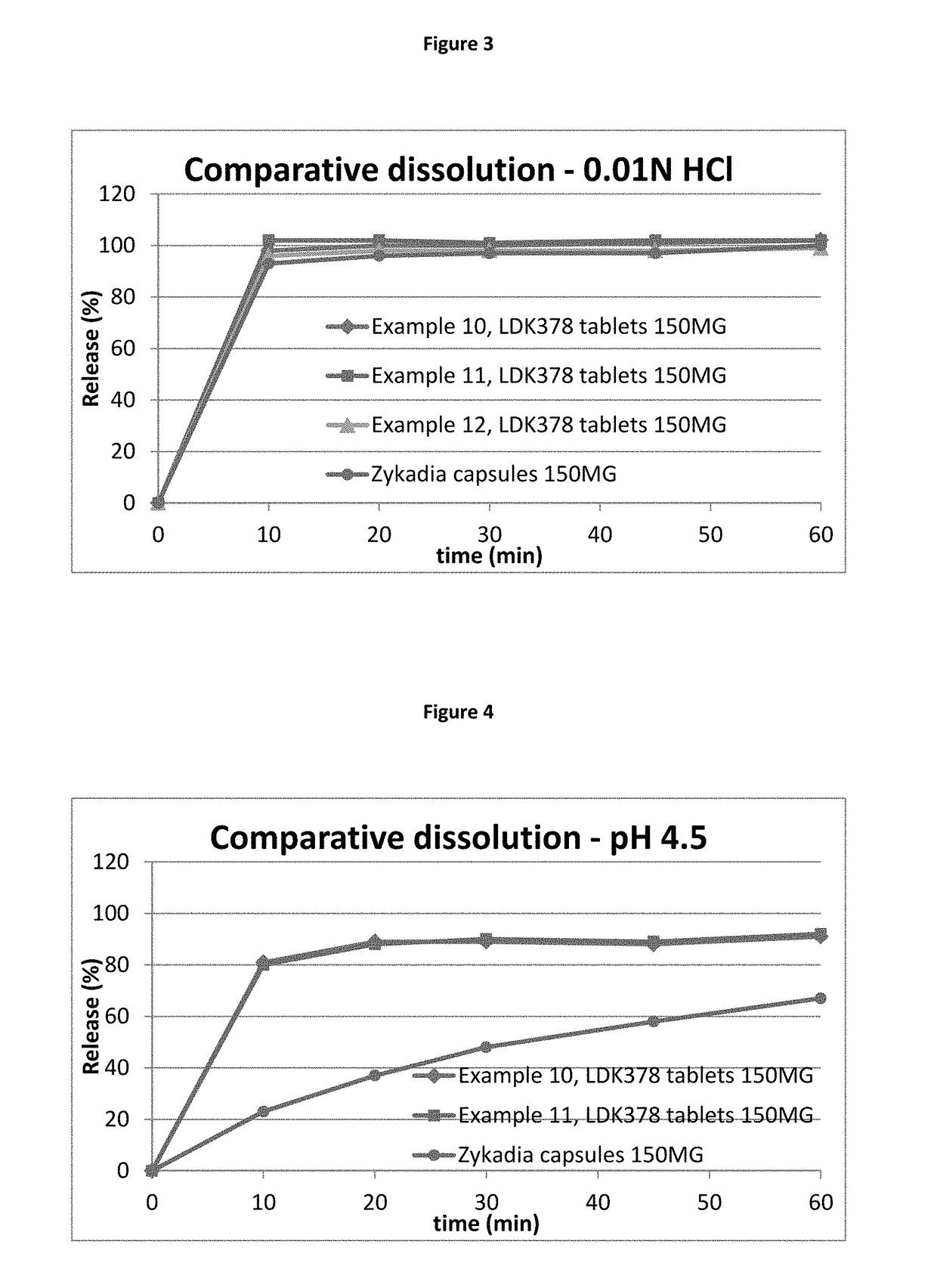
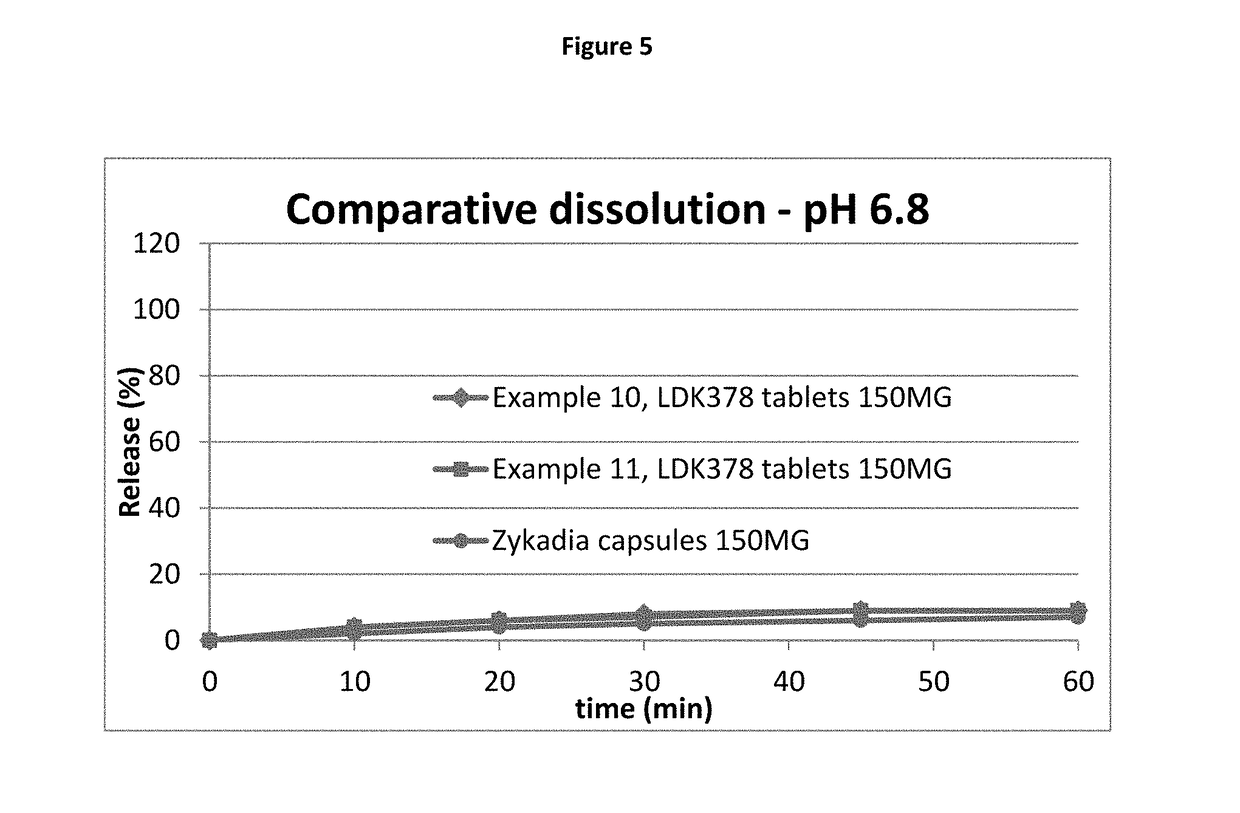
[0100]It was determined during the formulation preworks that all formulations tested showed very significant sticking issues during a tableting step. The sticking issue was occurring at all three tested common manufacturing processes for tablets, either direct blending / compression, roller compaction / compression or wet granulation / compression. However, the sticking issue was significantly reduced using wet granulation. In addition, the wet granulation approach allowed loading of the highest amount of drug. Roller compaction and direct compression did not result in an appropriate tablet or an acceptable manufacturing process, leading to the conclusion to that these technologies could not be selected. For direct compression (example 1) all listed excipients except magnesium stearate were blended, then Mg-stearate was added and the mix blended again and compressed.
[0101]Roller compaction approach (dry granulation) shown in example 2 was done by blending LDK378 drug sub...
examples 3b to 6
er
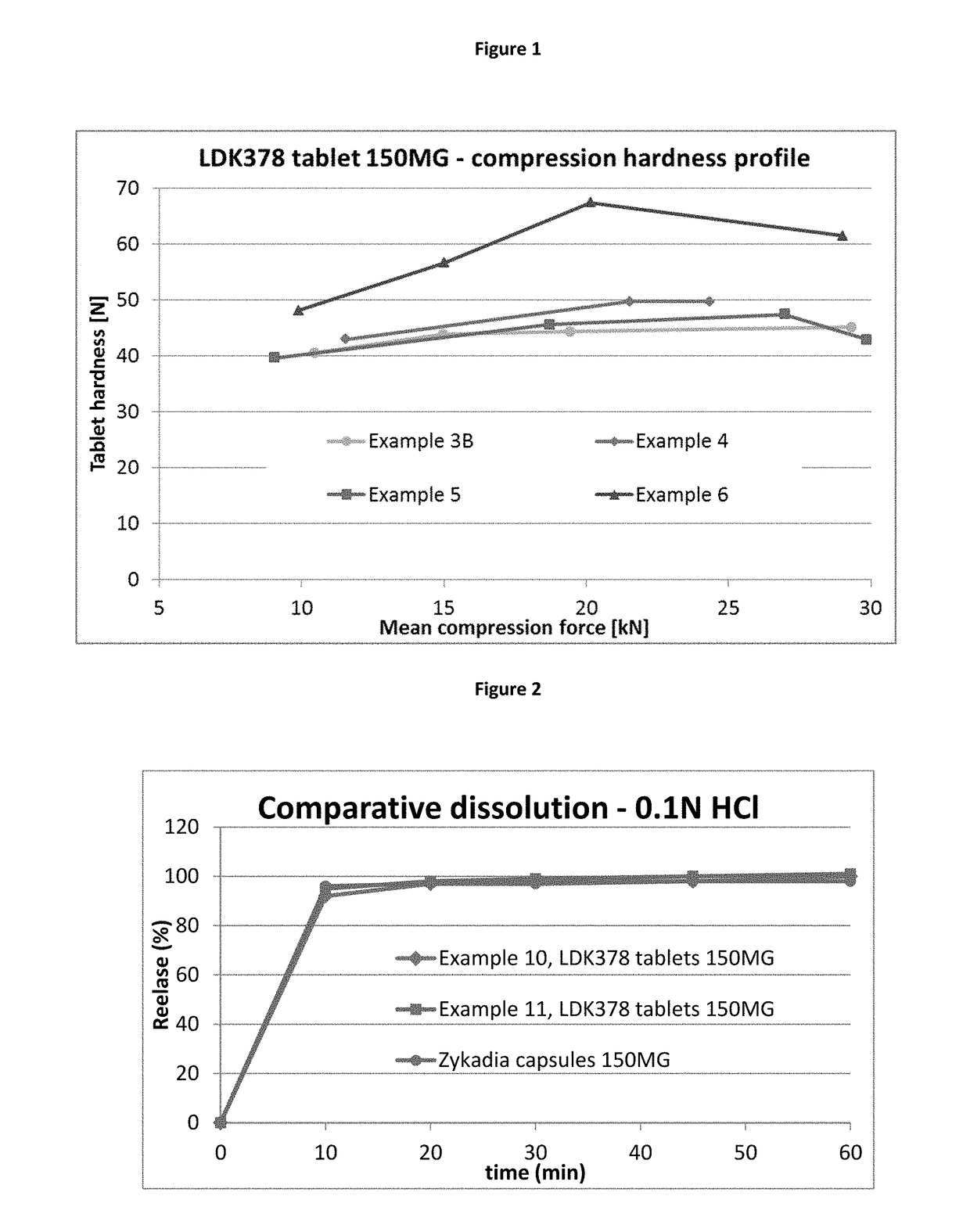
[0103]In an additional development step, the impact of the choice of a binder for a wet granulation phase was determined. Hypromellose and povidone were selected for testing out of several binder types (starch based binders, povidone based binders, copovidone based binders, hypromellose based binders, hydroxypropylcellulose based binders and hydroxyethylcellulose based binders) (cf. examples 4 and 5 in table 2). A binder for wet granulation can be added either in a dry state to the granulation mixture (granule phase) before the wet granulation is conducted with water or the binder can be dissolved in e.g. water to form a granulation liquid, which is then used to conduct the wet granulation of the granulation mixture. The granulation mixture can consist of a drug substance and several excipients (e.g. filler, disintegrant and / or other excipients) which are granulated together with the applied binder (examples 4 and 5). However, a specifically modified approach was taken, by which o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| total weight | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com