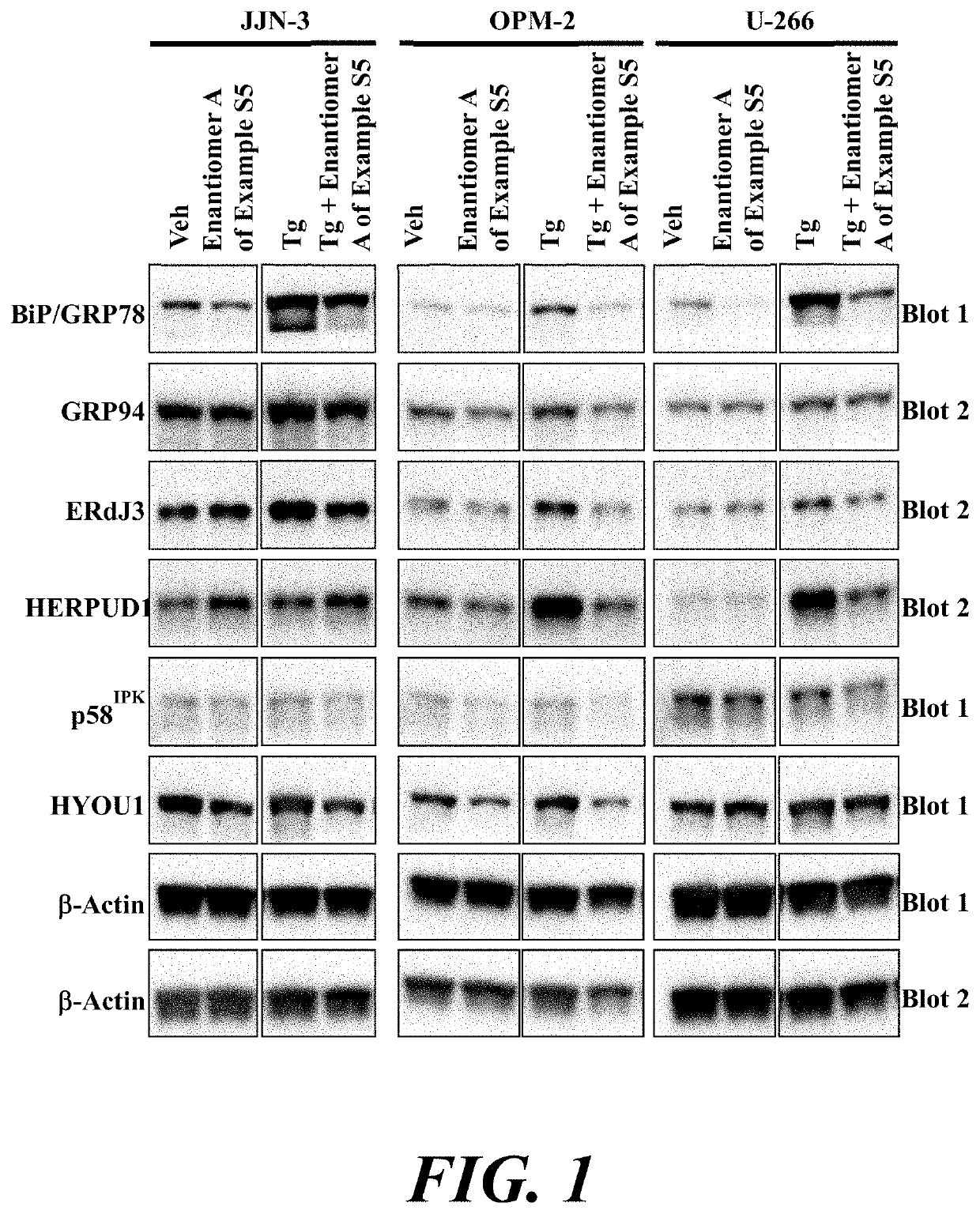
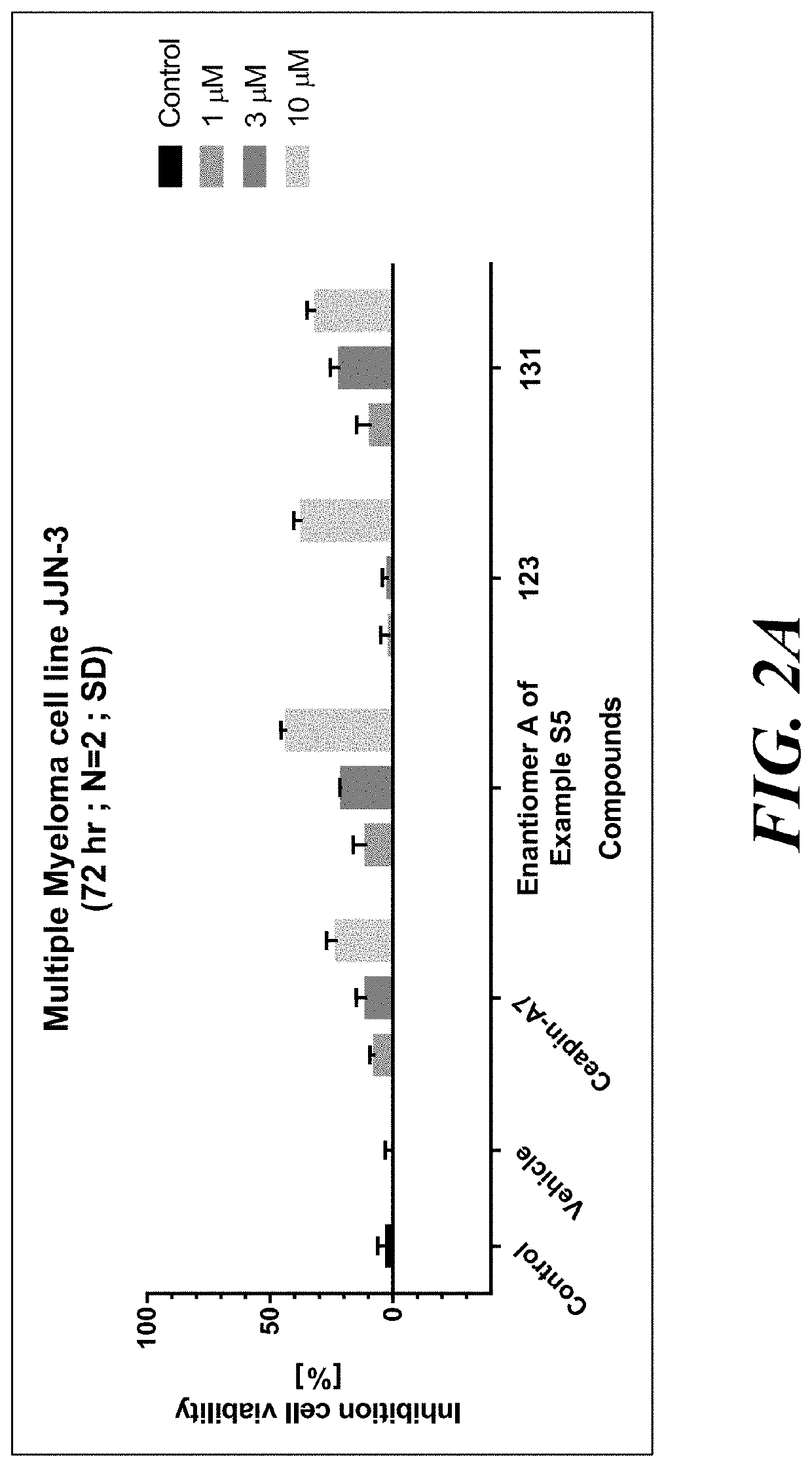
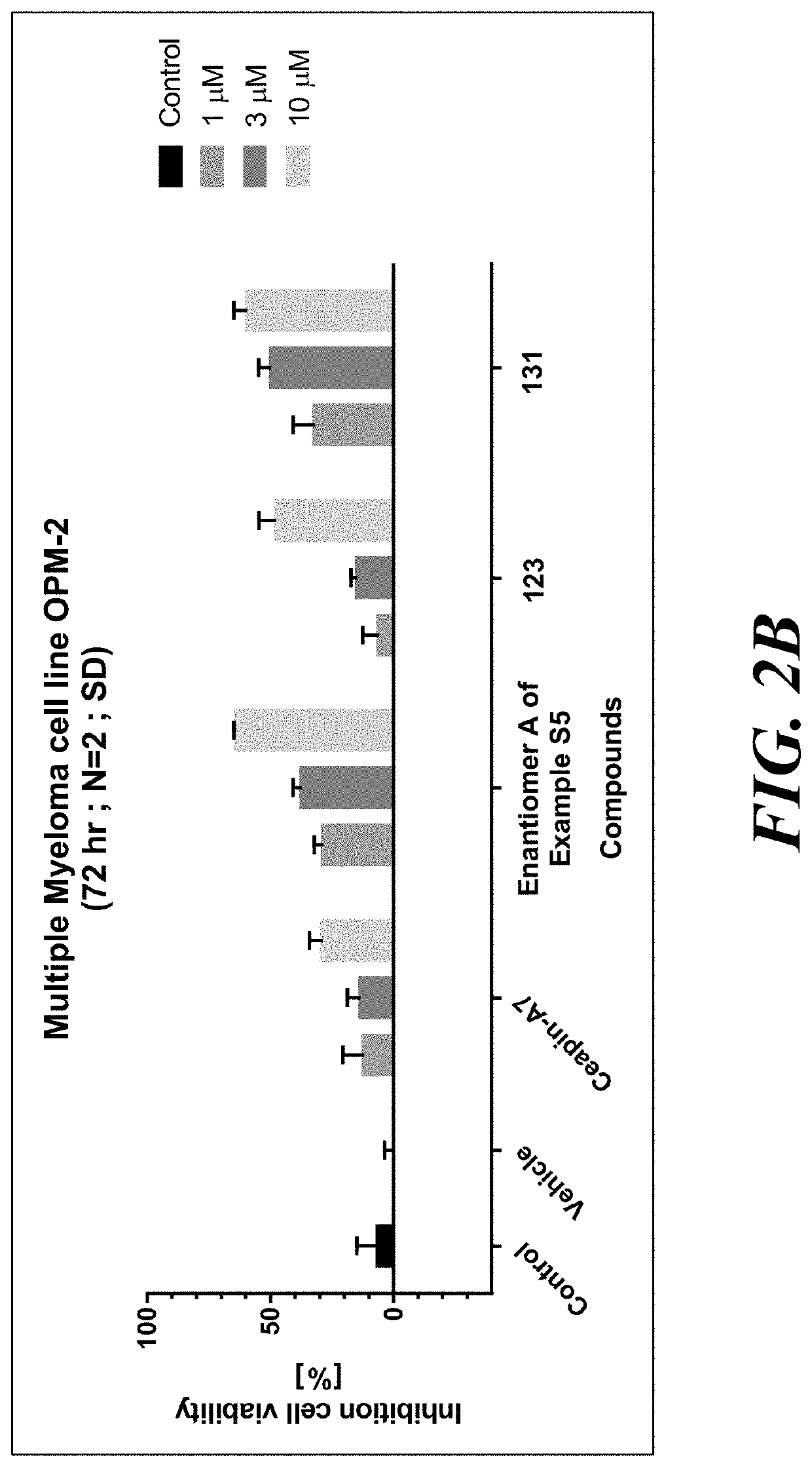
Atf6 inhibitors and uses thereof
a technology of atf6 and inhibitors, applied in the field of atf6, can solve the problems of cell death by engaging apoptosis, stress response, overflowing folding machinery,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Synthetic Examples
[0495]The following examples are offered to illustrate but not to limit the present disclosure. One of skill in the art will recognize that the following synthetic reactions and schemes may be modified by choice of suitable starting materials and reagents in order to access other compounds of Formula (A), (I), (Ia), (Ib), (Ic), (Id), (Ie), (If), (Ig), (Ih), (Ii), (Ij), or (Ik), or a salt thereof. The compounds are prepared using the general methods described above.
[0496]The following abbreviations are used throughout the Examples: DCM (dichloromethane), DIAD (diisopropyl azodicarboxylate), DIPEA or DIEA (N,N-diisopropylethylamine), DMF (N,N-dimethylformamide), DMSO (dimethyl sulfoxide), HATU ((1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate), HPLC (high-pressure liquid chromatography), IPA (isopropyl alcohol), LCMS (liquid chromatography mass spectrometry), NMR (nuclear magnetic resonance), PPh3 (triphenylphosphane), RT...
example s1
of N-(1-(1-(2,4-bis(trifluoromethyl)phenyl)propyl)-1H-pyrazol-4-yl)-5-(pyridin-2-yl)isoxazole-3-carboxamide (Compound 1)
[0497]
[0498]Step 1: Synthesis of 1-(2,4-bis(trifluoromethyl)phenyl)ethan-1-ol. To a stirred solution of 2,4-bis(trifluoromethyl)benzaldehyde (500 mg, 2.06 mmol, 1 equiv) in THF (5 mL) was added ethylmagnesium bromide (412 mg (3 ml), 3.09 mmol, 1.5 equiv) portion wise at rt, and the reaction was stirred for 10 minutes. The reaction mixture was allowed to stir for 1 hour at RT. Product formation was confirmed by TLC & NMR. After completion of the reaction, the reaction mixture was quenched with water and extracted with ethyl acetate (50 mL×3). The combined organic extracts were washed with water (50 mL×2), dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain 1-(2,4-bis(trifluoromethyl)phenyl)ethan-1-ol. (110 gm, 19% as colourless liquid). 1H NMR (400 MHz, DMSO-d6) δ=8.13-8.06 (m, 1H), 8.05-7.99 (m, 1H), 7.94 (s, 1H), 4.80 (br. s., 1H), 1.65-1...
example s2
of N-(7-(2,4-bis(trifluoromethyl)benzyl)-1H-pyrazol-4-yl)-5-(2-hydroxypropan-2-yl)isoxazole-3-carboxamide (Compound 2)
[0502]
[0503]Step 1: Synthesis of 1-(2,4-bis(trifluoromethyl)benzyl)-4-nitro-1H-pyrazole. 4-nitro-1H-pyrazole (0.73 g, 0.006 moles, 1 eq) was taken in DMF (20 mL). Cool this reaction mixture by ice water up to 0° c. add K2CO3 (1.34 g, 0.009 mole, 1.5 eq) portion wise in it stirred reaction mixture for 10 minute and then add 1-(bromomethyl)-2,4-bis(trifluoromethyl)benzene (2.0 g, 0.006 mole, 1 eq) in it by drop by drop. Stir above reaction mixture for 1 hour (reaction was monitored by TLC & LCMS). After completion of reaction, reaction mixture was diluted with ethyl acetate (50 mL) and extracted with water (50 mL). Collect organic layer and concentrate it to obtain product which further purified by flash chromatography to obtain White color product. LCMS: 339 [M+H]+.
[0504]Step 2: Synthesis of 1-(2,4-bis(trifluoromethyl)benzyl)-1H-pyrazol-4-amine. To a stirred solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com