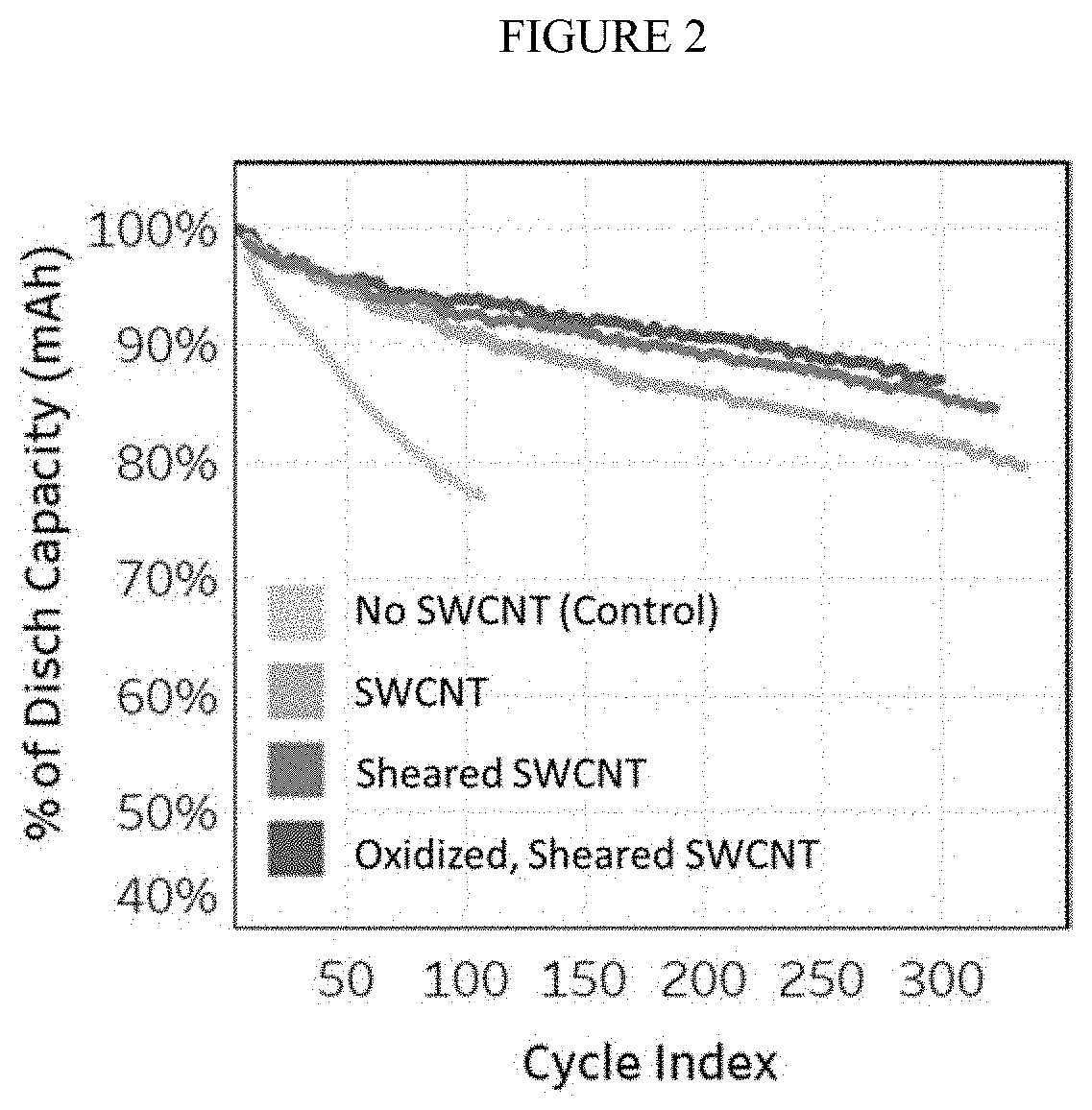
Dispersions comprising high surface area nanotubes and discrete carbon nanotubes
a technology of carbon nanotubes and nanotubes, which is applied in the direction of electrically conductive paints, applications, transportation and packaging, etc., can solve the problems of affecting the use of carbon nanotubes in these applications, and tubes with low aspect ratios not suitable for high strength composite materials, etc., to achieve improved battery capacity and power, improve electrical and ionic conductivity, and reduce practical specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Tuball™ (OCSiAl)
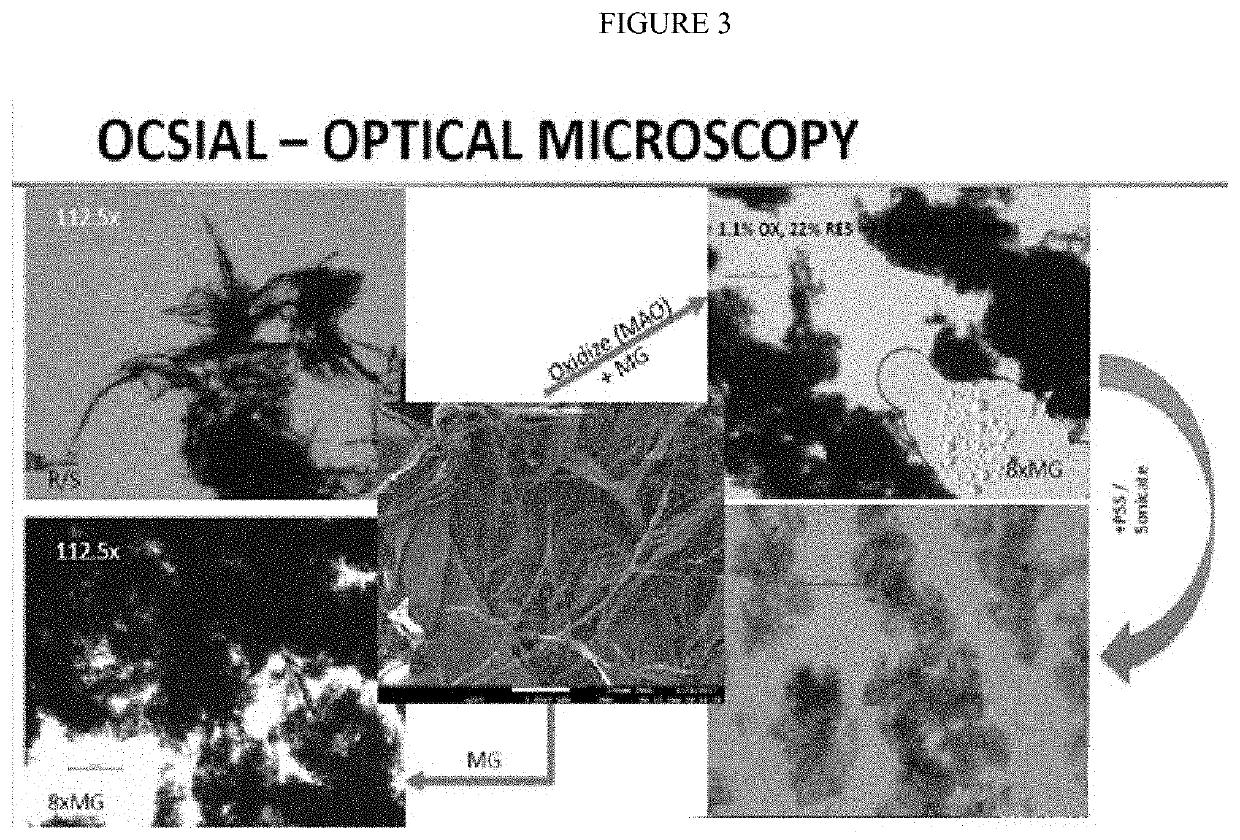
[0270]Thirty-five grams of >64% nitric acid is heated to 95 degrees C. To the acid, 15 grams of as-received, single-walled carbon nanotubes (Tuball™) are added. The as-received tubes have the morphology of tightly bundled tree-trunks. The mixture of acid and carbon nanotubes are mixed while the solution is kept at about 95 degrees Celsius for 5 hours and is labeled “oSWCNT-82-2”. At the end of the reaction period, the oSWCNT 82-2 are filtered to remove the acid and washed with reverse osmosis (RO) water to pH of 3-4. The resulting CNTs were oxidized to about 3.6% and contained about 4.4% metal residue.
[0271]Variations on this process were also conducted using slightly differing parameters as shown below in Table 1:
[0272]Samples oxidized by an acid process: e.g. 35 g HNO3 (65%) / 15 g Tuball™, 95° C. oxidation.
[0273]23.33 g HNO3 (65%)+10.01 g CNT. T=95° C. Initial big plume of NOx at addition of CNT.
TABLE 1Time (hr)T(° C.)% Ox% Res094.21.1121.7195.62.5295.62.44.5395.62....
example 2
atment of Non-Oxidized and Oxidized OCSiAl Tubes
example 2a
tment of Oxidized OCSiAl Tubes
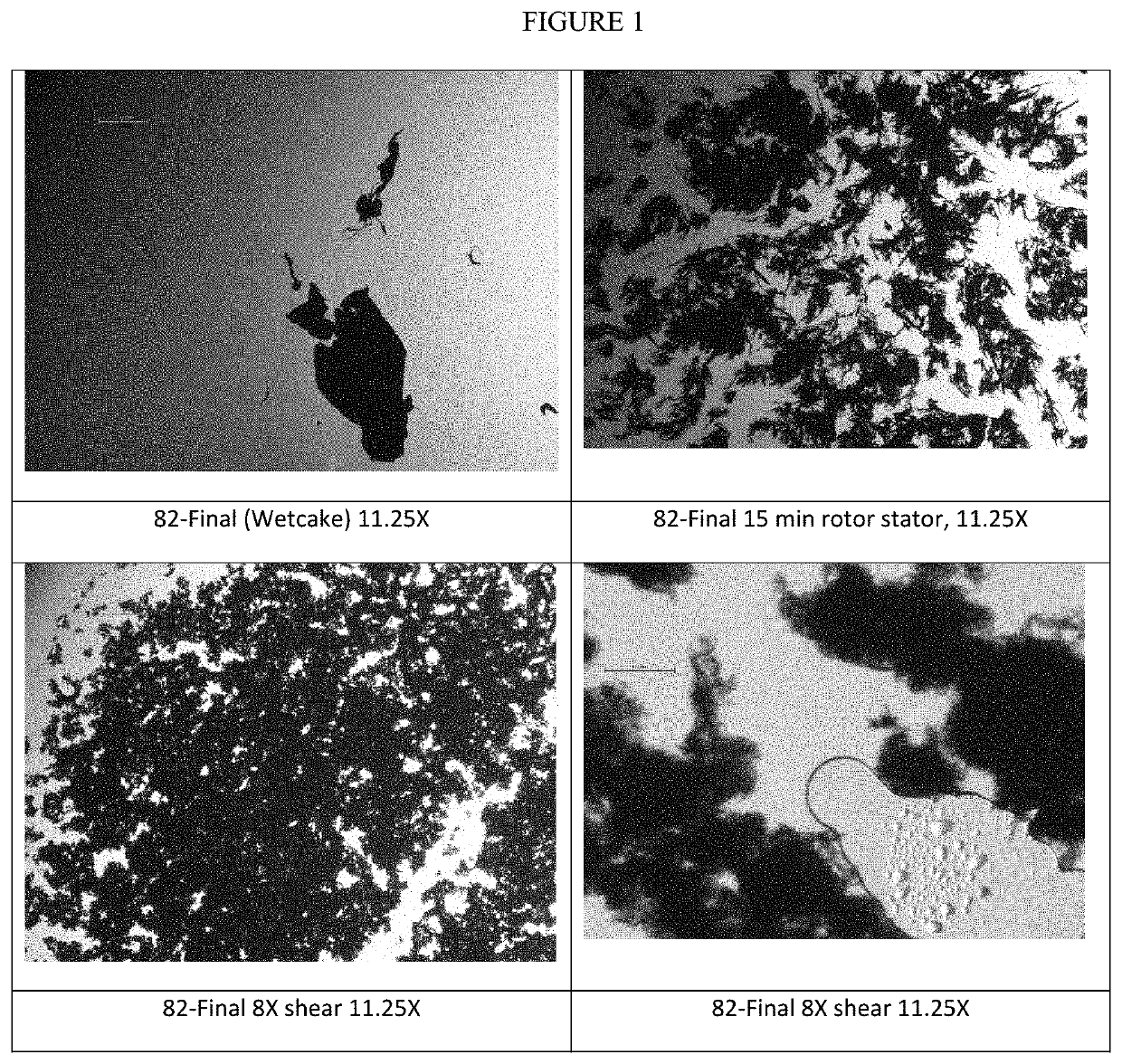
[0280]Sample volume ˜1200 mL. Use 1.5 L stainless steel container for Rotor / Stator (R / S) work.
[0281]Oxidized OCSiAl ˜0.15%
[0282]Oxidized OCSiAl source: 82-final (pH 3.61, 27.1% solids)
[0283]1200 g×0.15%=1.8 g dry equiv.=6.64 g wetcake. Used 6.65 g wetcake.
[0284]Check viscosity through Rotor Stator (R / S) as shown below.
T (min)T (° C.)Comments023531Clear liquid droplets on plastic coveringvessel opening. Not viscous941Clear liquid droplets on plastic coveringvessel opening. Not viscous+6.62 g wetcake1550Viscous mixture. Proceed to shearingPlace in Freezer for ~1.5 hr.
[0285]Shearing
Pass #T (° C.)Comments1251500 psi because noticed some large particlespresent when cleaning the rotor stator236342Place in freezer 45 minutes → 15° C.4315376457511 hr freezer → 25° C.839Sample for optical microscopy
[0286]Sample name 180417-MF-1A (0.26% solids), 180417-MF-1B (0.22% solids) 19 g.
[0287]Optical Microscopy, shown in FIG. 1, shows a progression from wetcake to rotor s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com