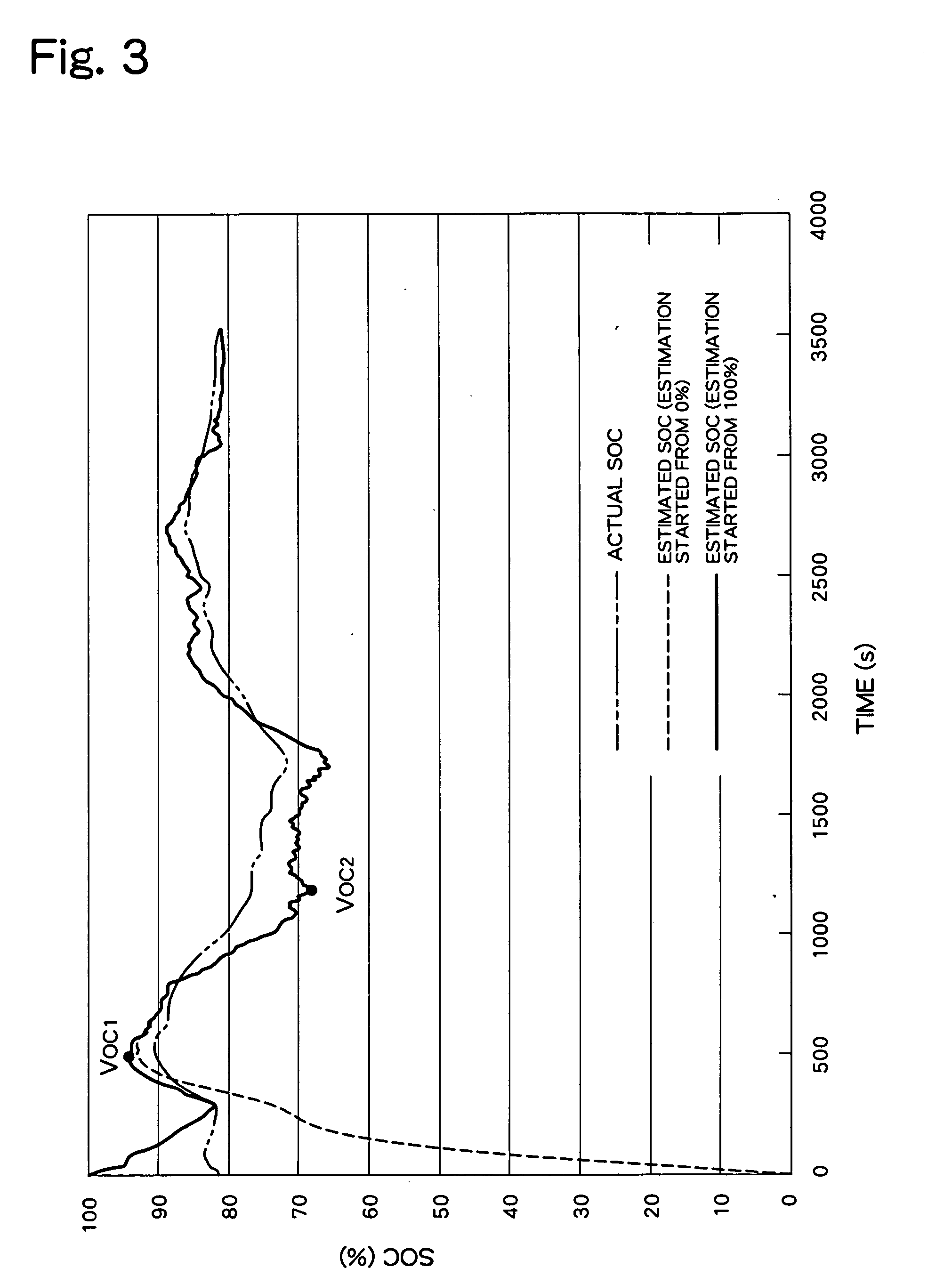
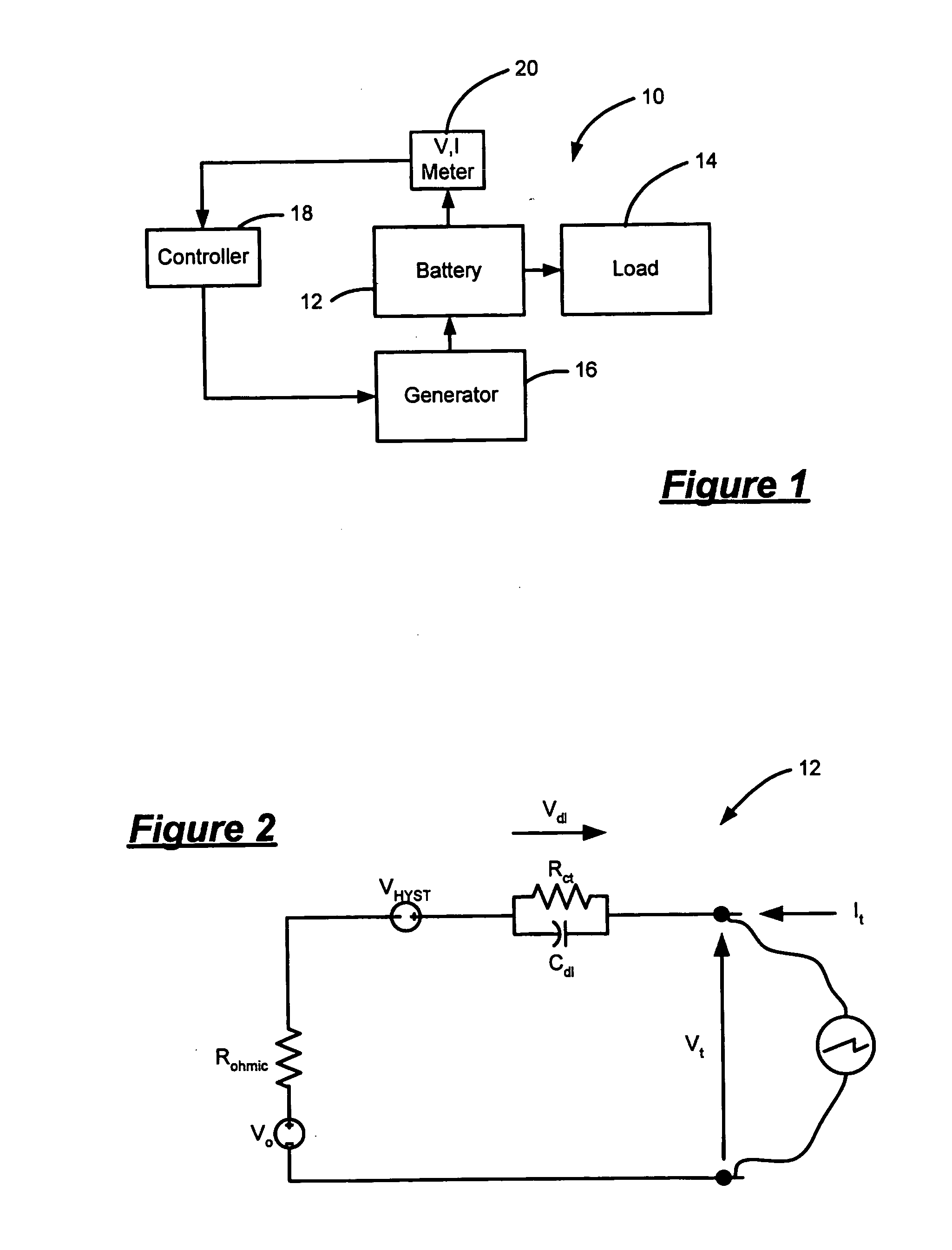
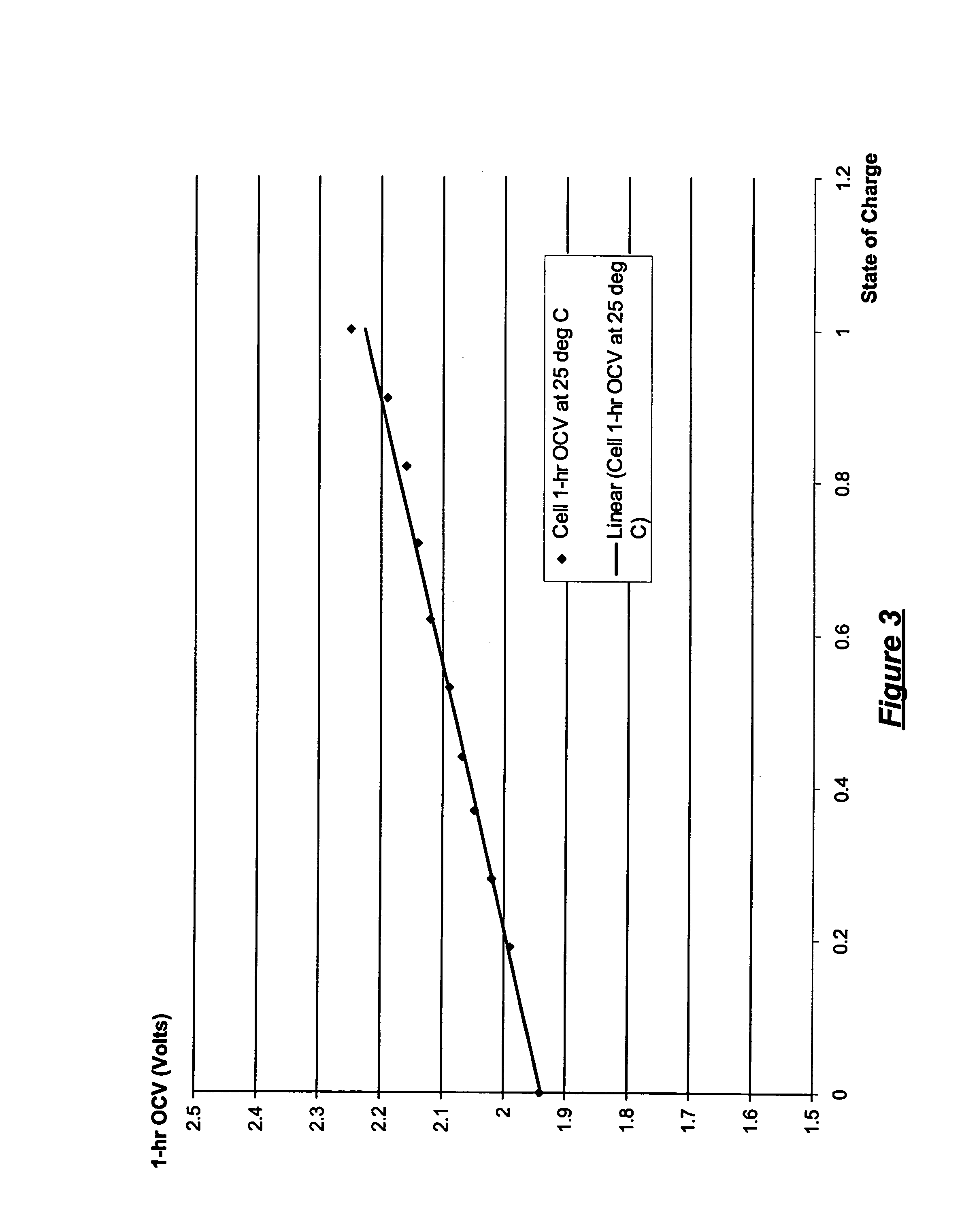
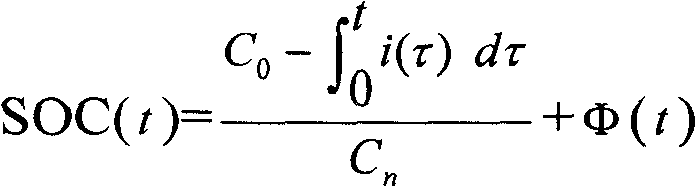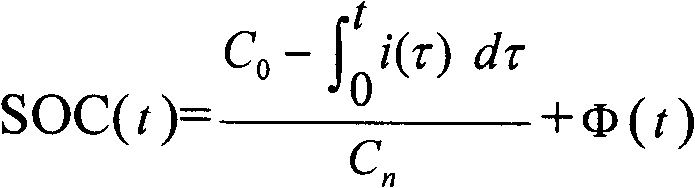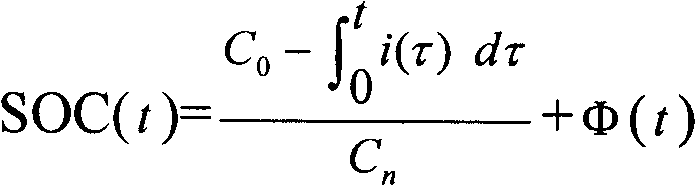Patents
Literature
722 results about "Battery state of charge" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
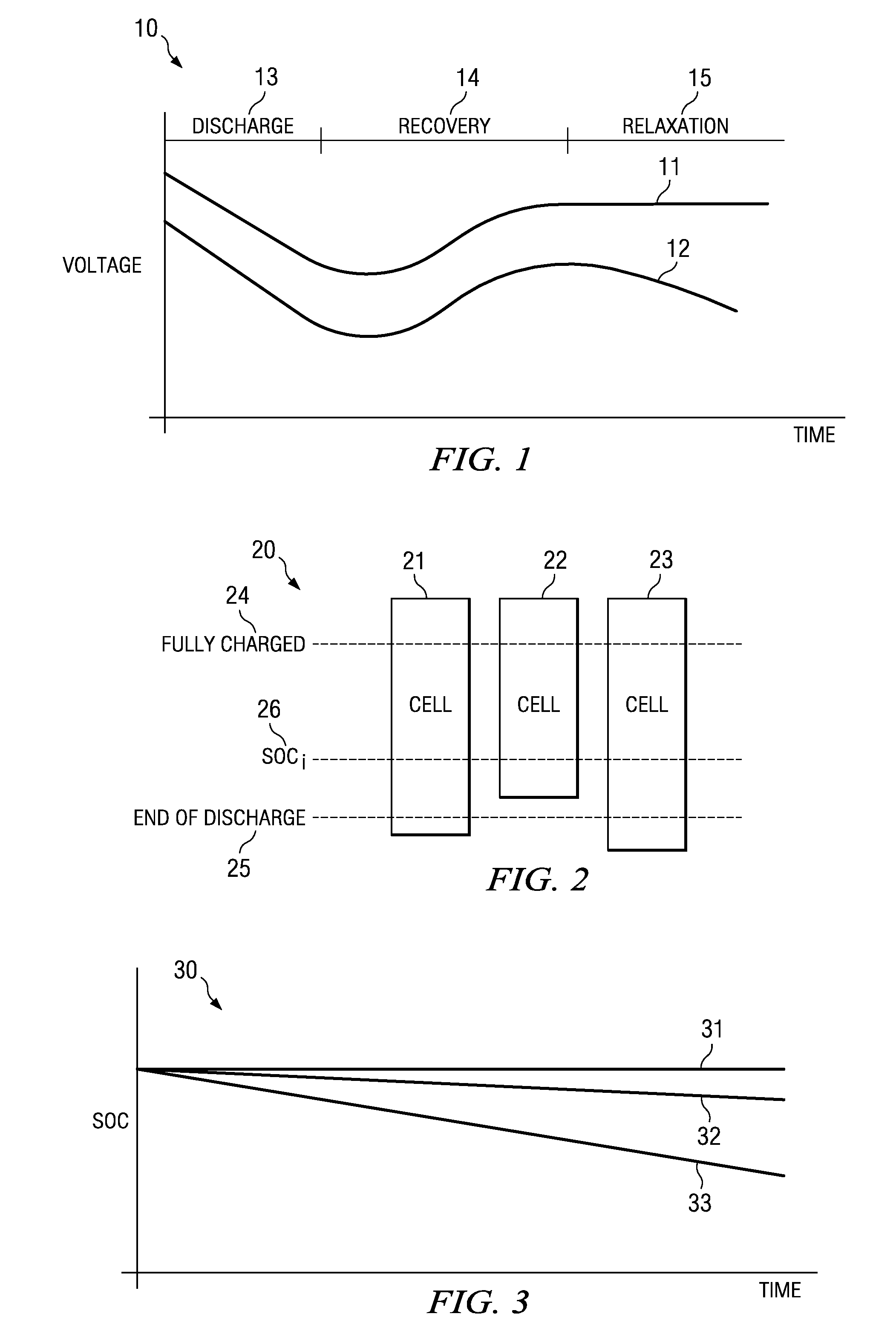
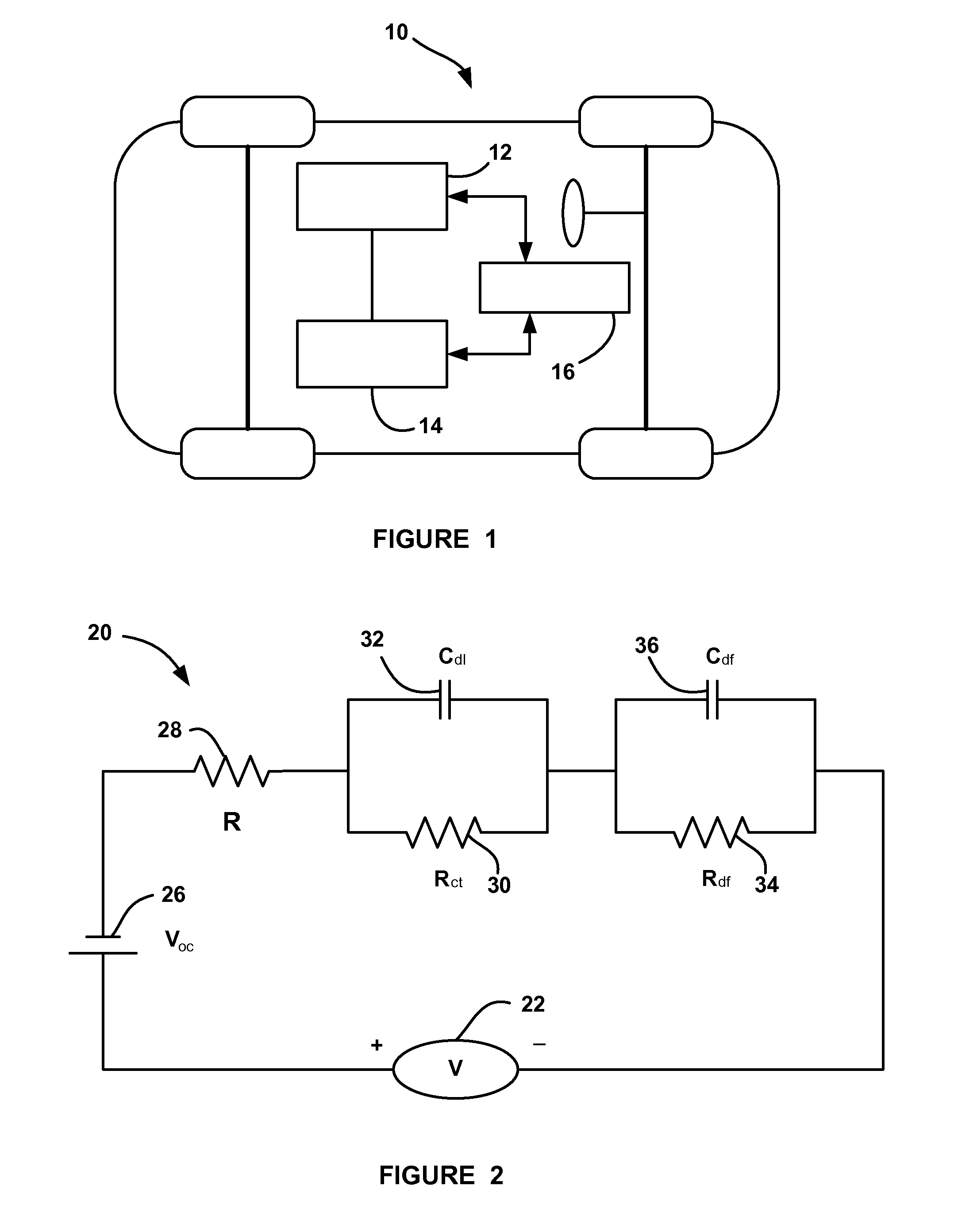
The ‘State Of Charge’ (SOC) of a battery is a measurement of how much energy is remaining (percent). It’s like a fuel gauge. Measuring and knowing the SOC of a battery or battery bank is useful when applying towards alternative energy, or any other situation where you need to know its condition.
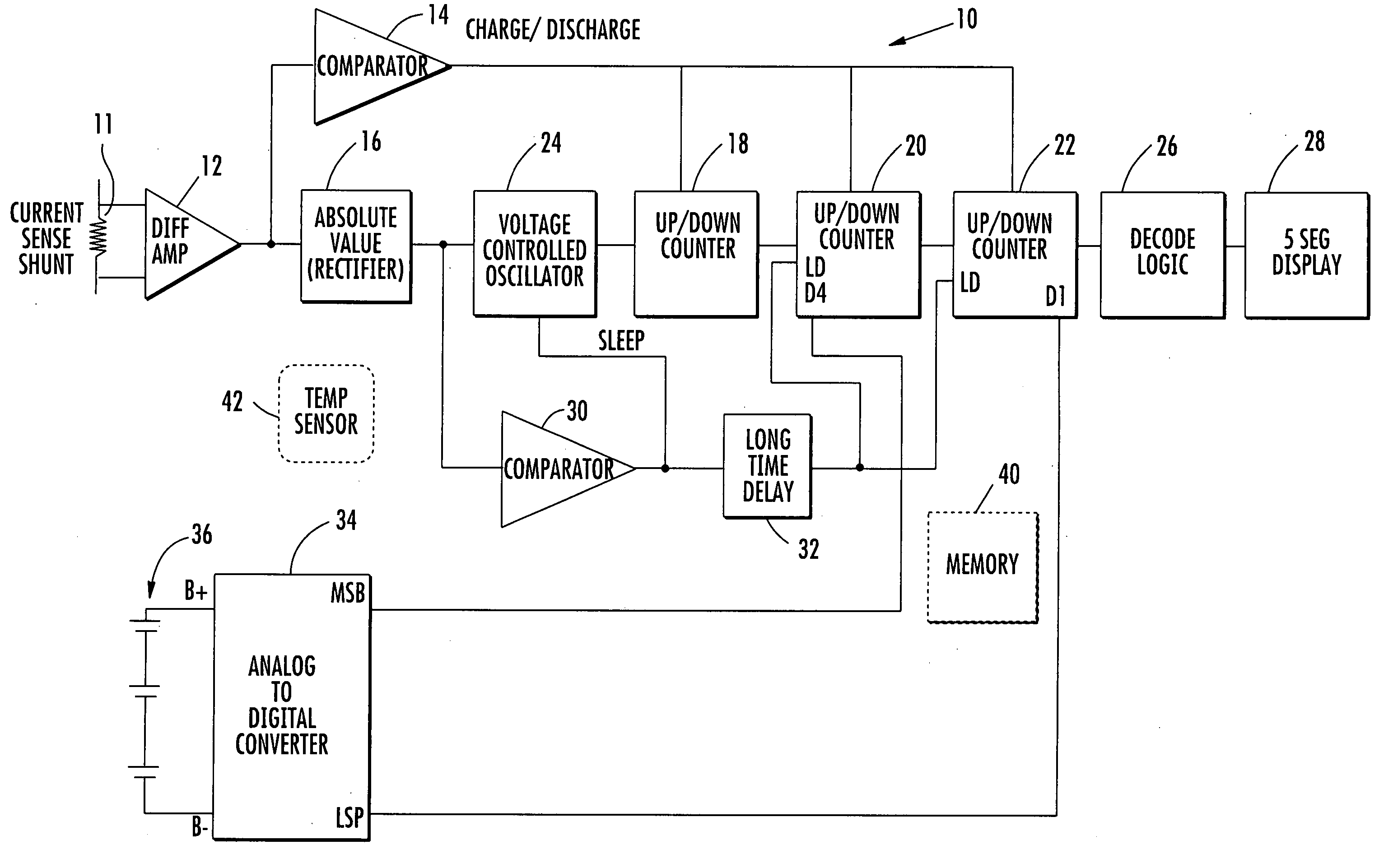
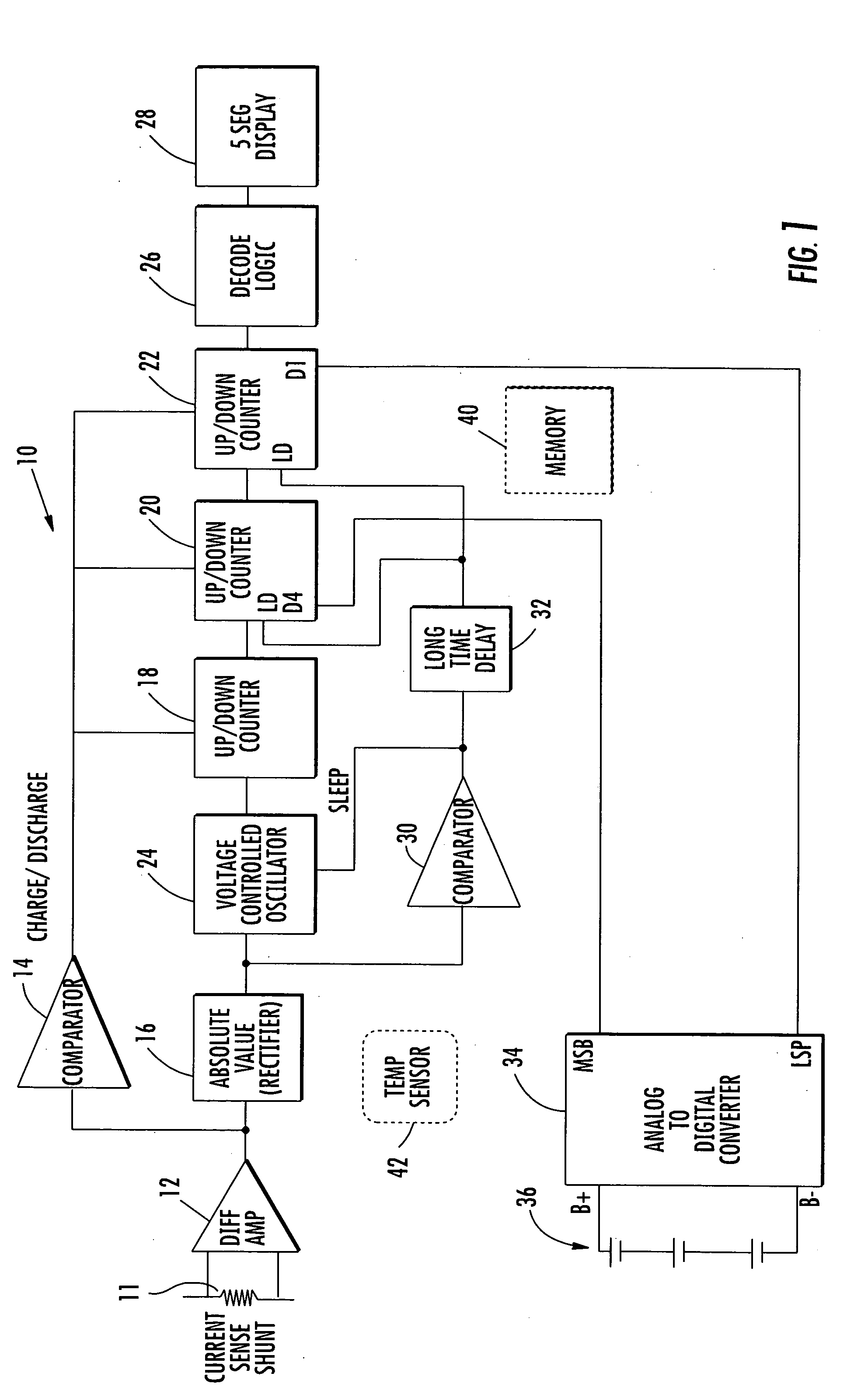
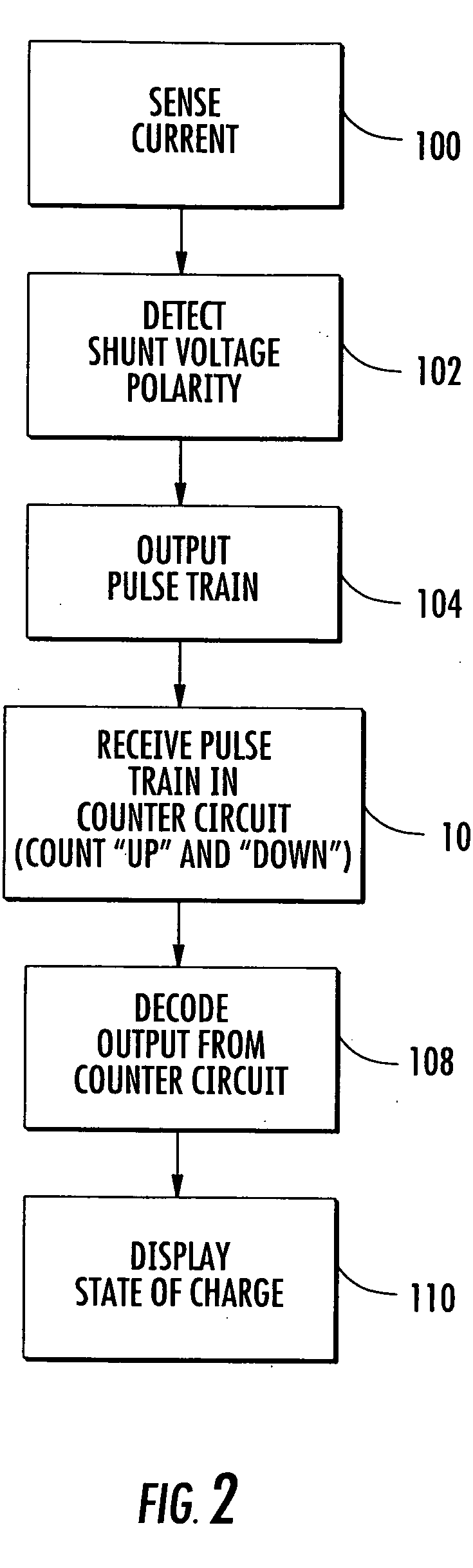
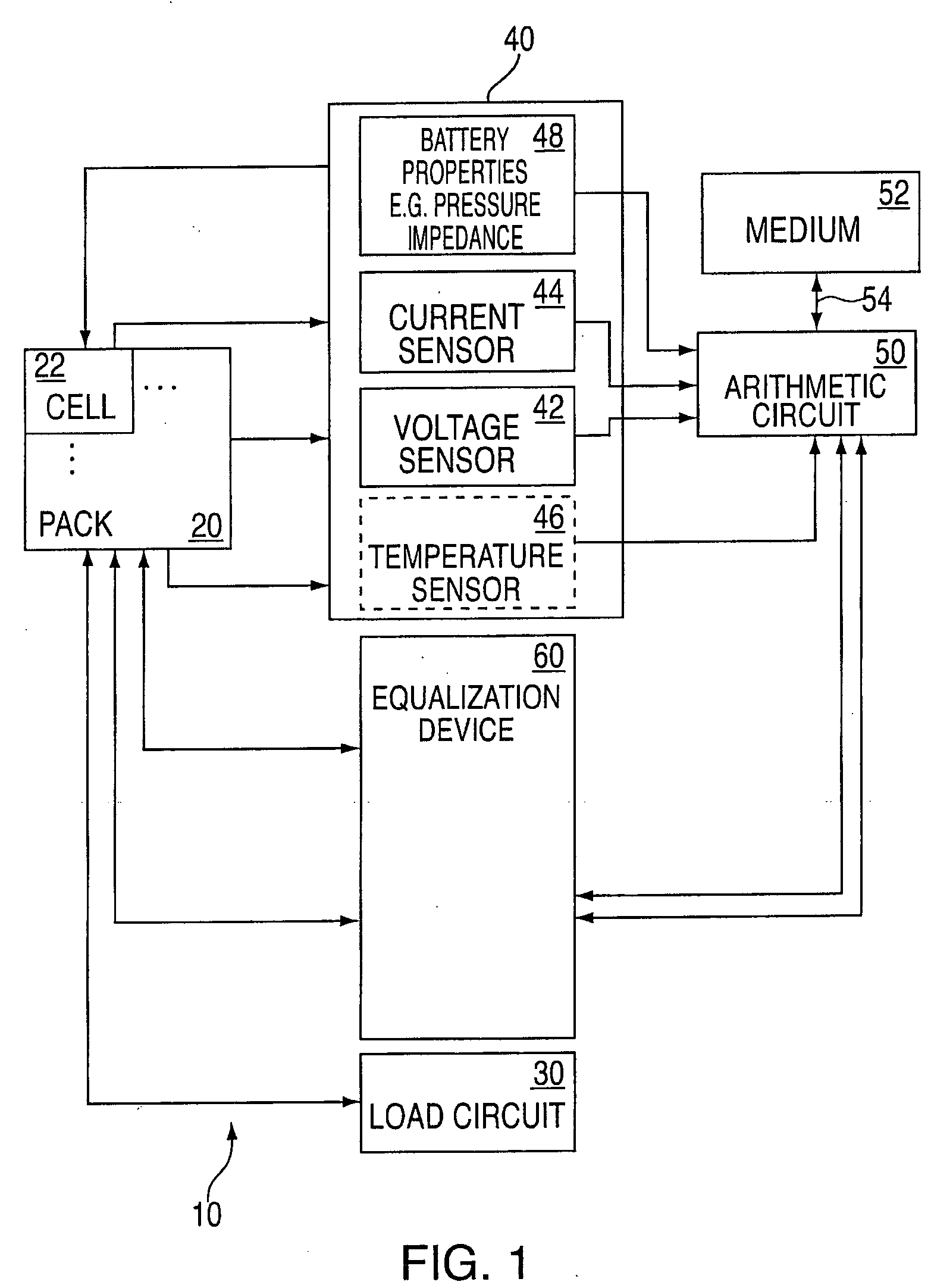
State of charge indicator for battery
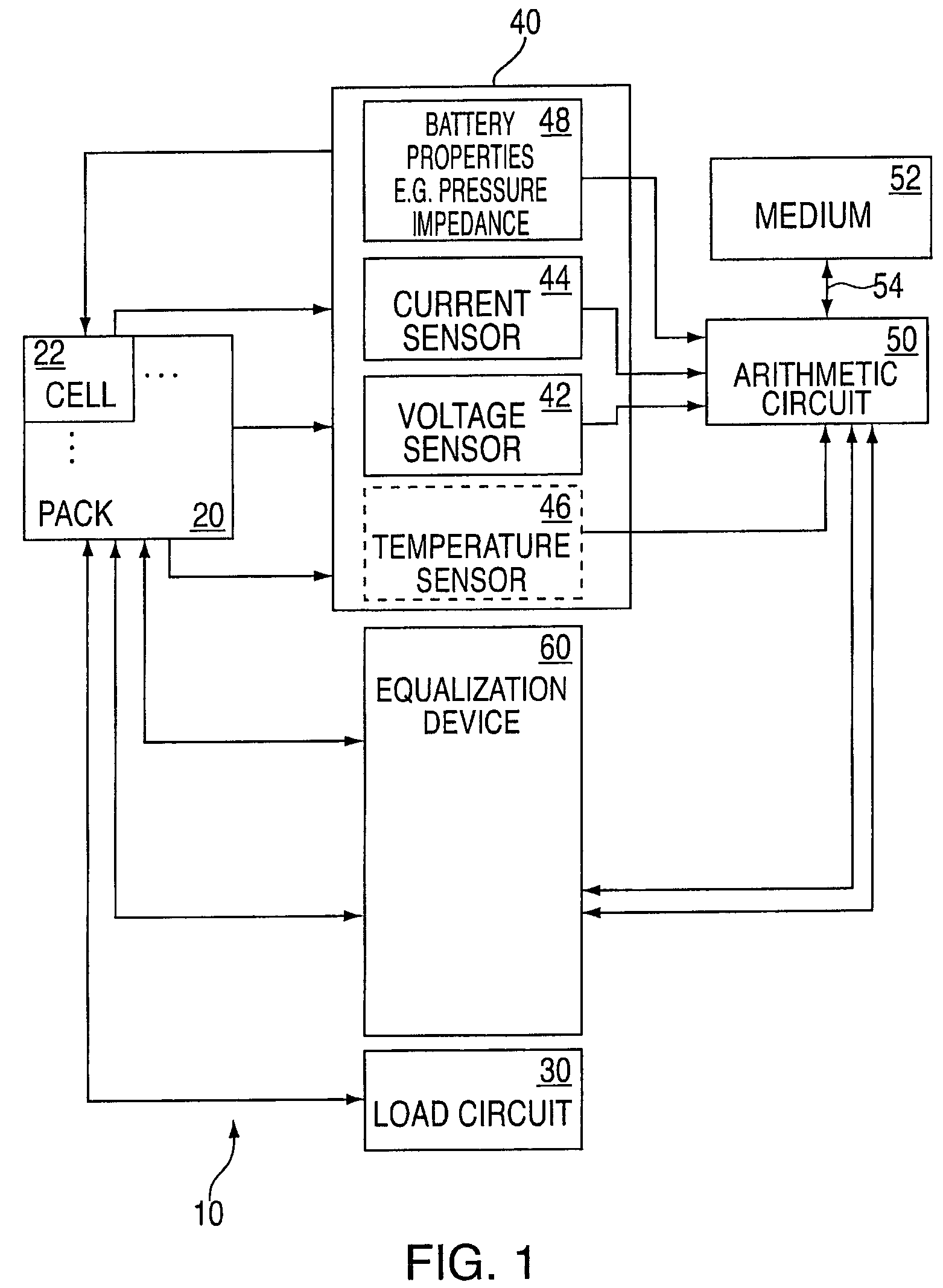
InactiveUS20060097699A1Circuit monitoring/indicationCharge equalisation circuitBattery state of chargeBipolar voltage
A state of charge indicator includes a current sensing circuit for sensing and converting charge or discharge current of a battery into a bipolar voltage. A counter circuit counts battery charge. A charge / discharge circuit is operatively connected to the current sensing and counter circuits and detects the voltage polarity from the current sensing circuit and sets the counter circuit to a count mode with an up or down count for a respective charge or discharge. A reset circuit is operative with the current sensing circuit, counter circuit and charge / discharge circuit for resetting the counter circuit to an actual state-of-charge of the battery after delay when the battery is idle representative of a battery open circuit voltage.
Owner:MATHEWS ASSOCS
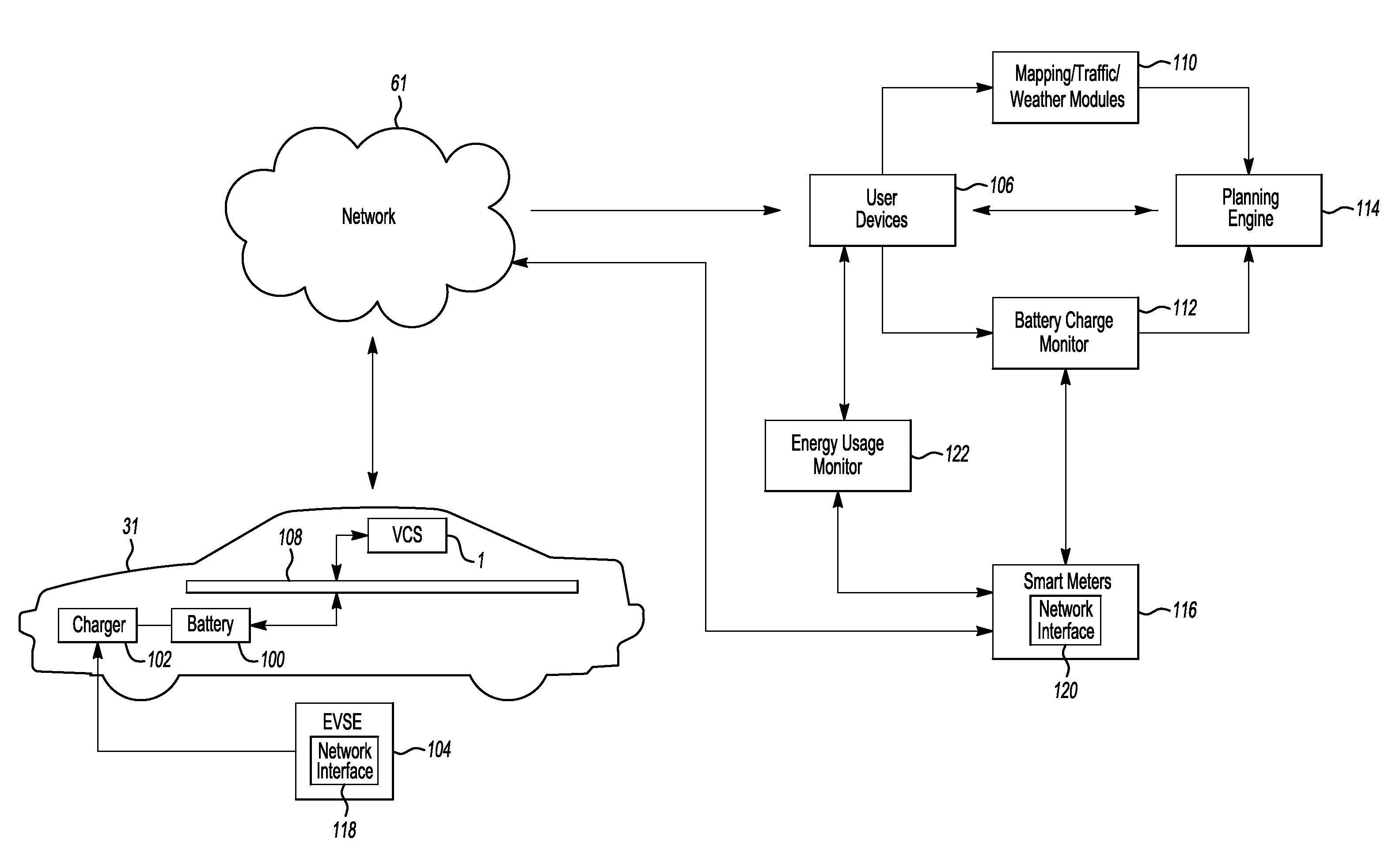
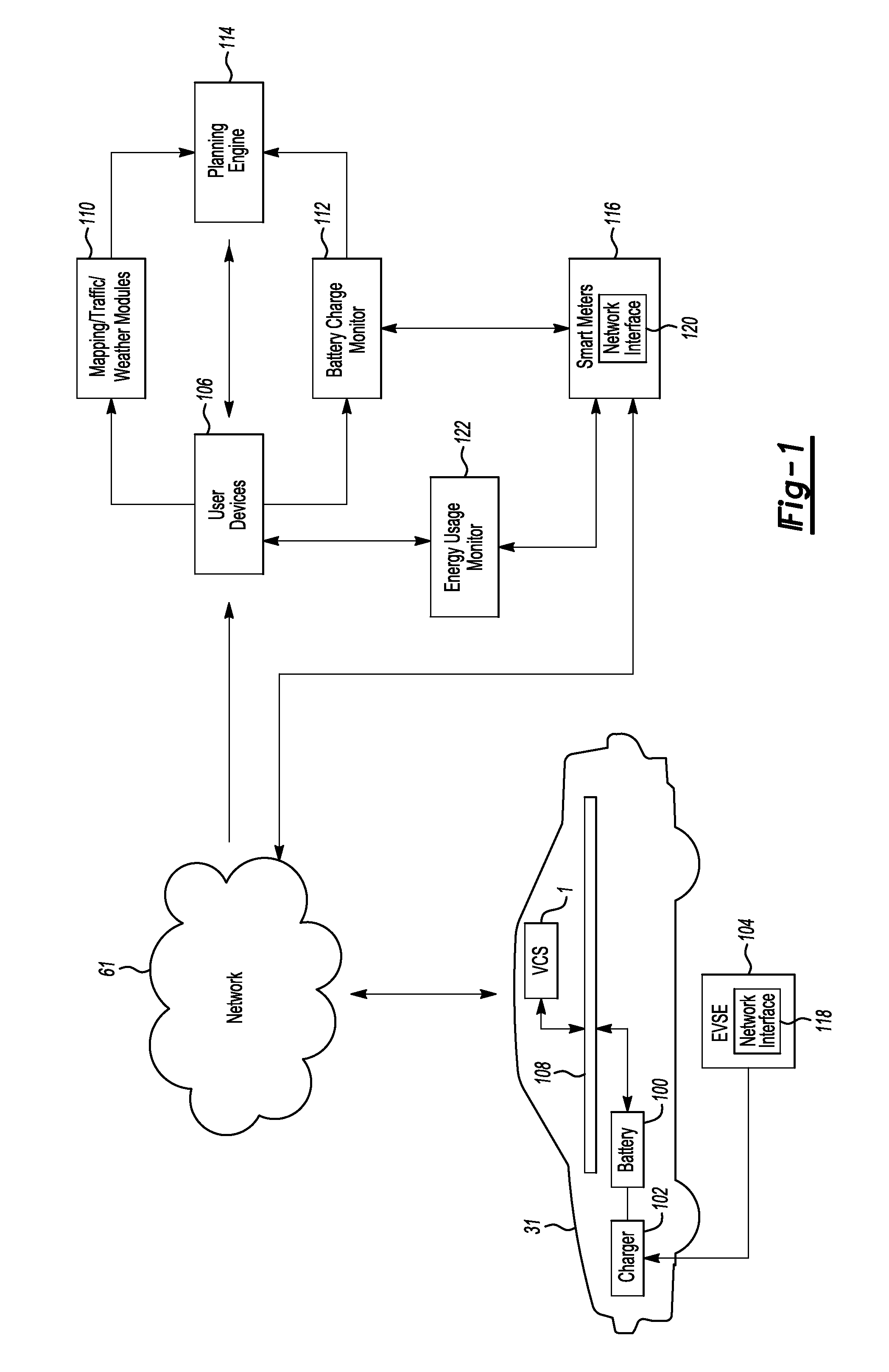
Method and system for monitoring an energy storage system for a vehicle for trip planning
One or more embodiments may include a trip planning system for planning a trip based on the charge of an electric vehicle battery. The trip planning system may include one or more computers located remotely from an electric vehicle which may have one or more battery packs for powering the electric vehicle. The computer(s) may be configured to receive geographic parameters defining a trip and a battery charge status of the one or more electric vehicle battery packs. The computer(s) may be further configured to determine that the trip cannot be completed based on the battery charge status. The computer(s) may additionally be configured to present a battery charge requirement for completing the trip.
Owner:FORD GLOBAL TECH LLC
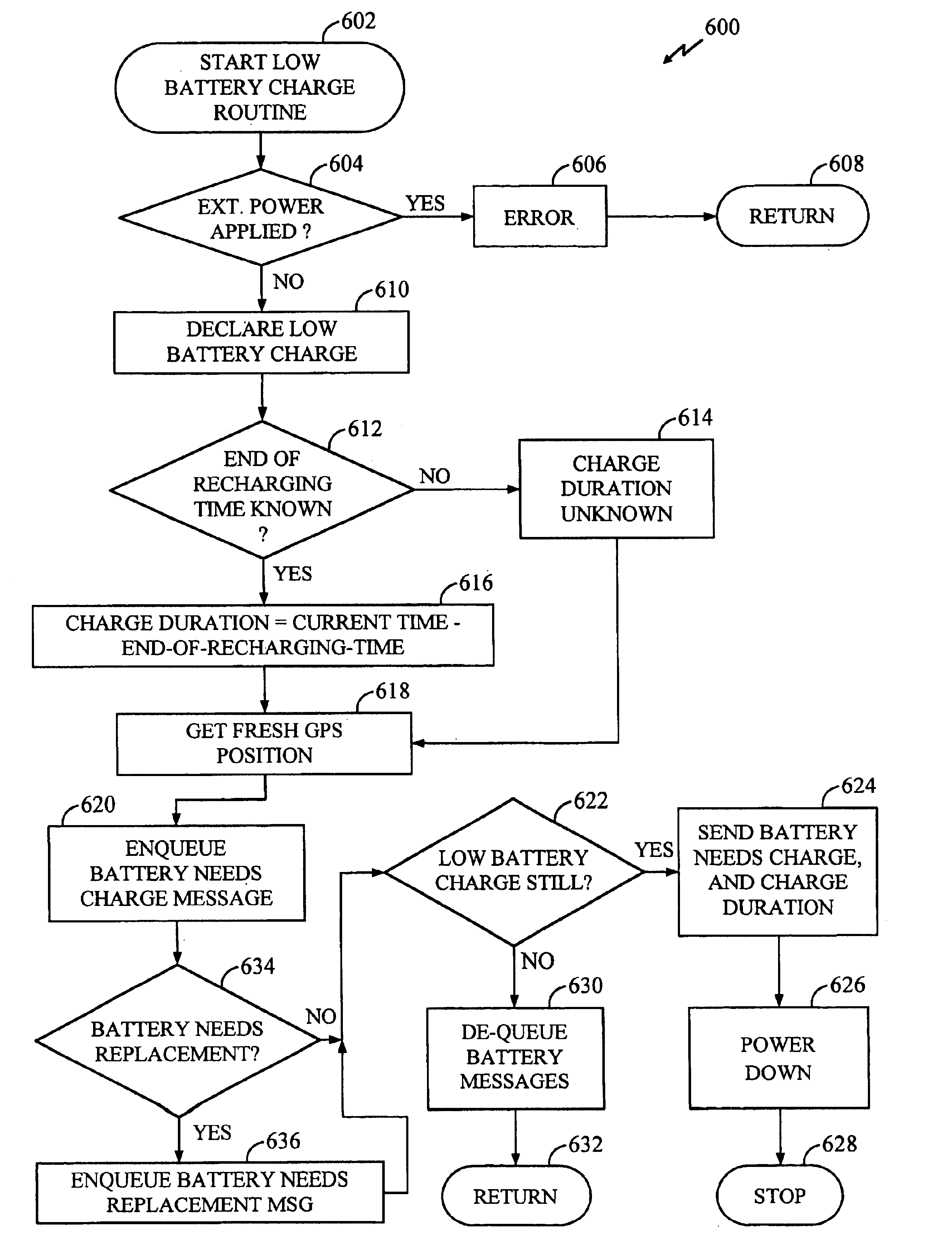
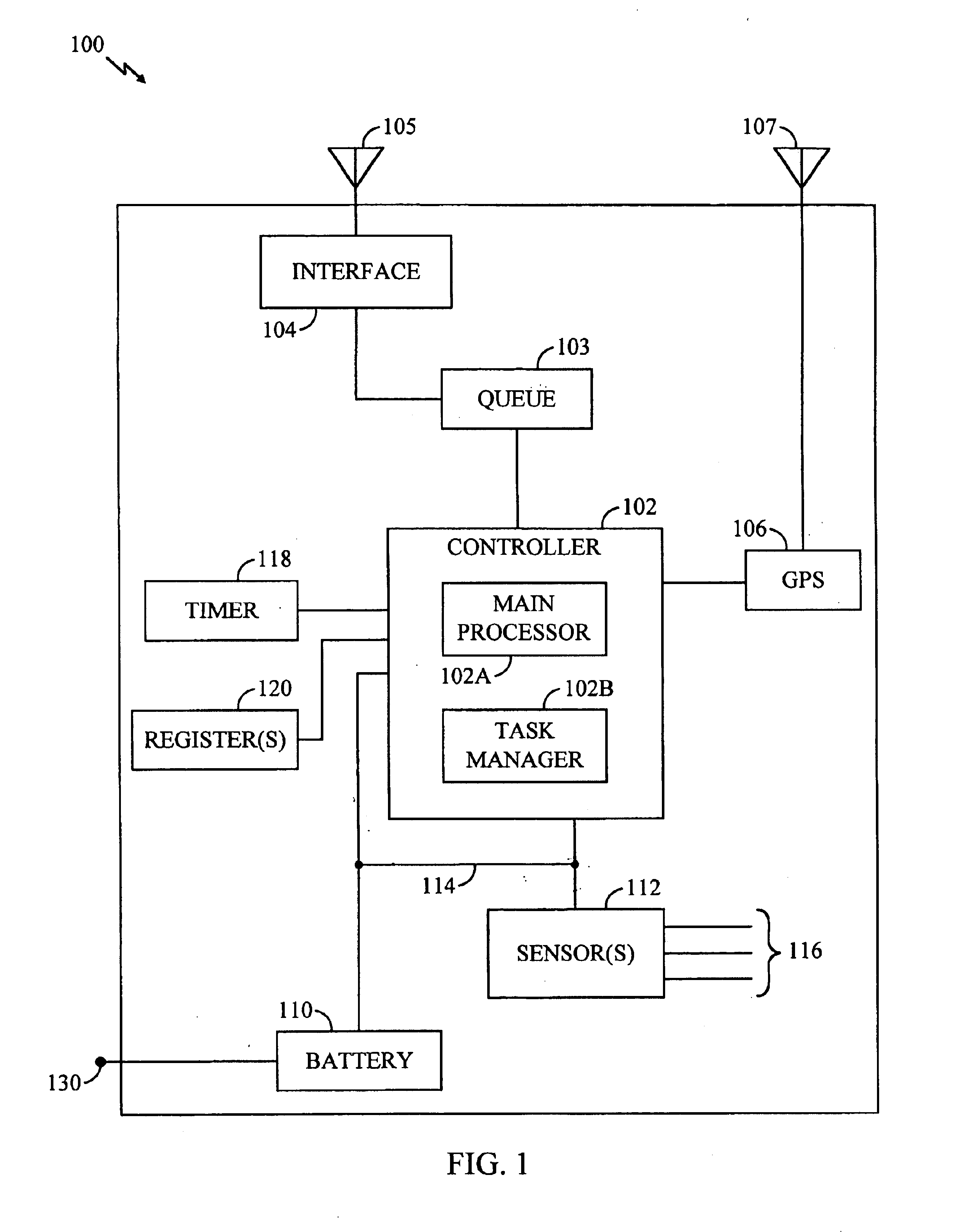
Battery monitoring system with low power and end-of-life messaging and shutdown
InactiveUS7024321B1Improve abilitiesLow costCircuit monitoring/indicationDifferent batteries chargingBattery state of chargeBattery charge
A battery monitoring apparatus that senses battery conditions such as low battery charge, end of battery capacity, and end of battery life, and responds by taking actions such as sending messages to a remote site and / or powering down. A rechargeable battery is coupled to one or more power-consuming electrical components, including battery monitoring equipment. The battery monitoring equipment senses battery charge. In response to a low-battery-charge condition, the battery monitoring equipment transmits a battery status message to a remote site and powers-down some of the electrical components. Whenever the battery nears the end of its capacity, the monitoring equipment powers down all electronic components and awaits the application of external power. The invention also tracks the time required for the battery charge to deplete. Charge duration decreases over time, and whenever it reaches a predetermined minimum, the battery monitoring equipment transmits a representative status message to the remote site.
Owner:OMNITRACS
Prioritized-routing for an ad-hoc, peer-to-peer, mobile radio access system
InactiveUS6873839B2Minimizes RF energyReduce distractionsEnergy efficient ICTPower managementBattery state of chargeBattery charge
An ad-hoc, peer-to-peer radio access system having a series of remote terminals, where each remote terminal is capable of forming a link, or hop, of the routing of a call made by one of the series of terminals. The status of the battery of each terminal which may potentially form part of the routing path of a call is reported to other terminals, whereby the routing path for a call will be decided also based on the status of the battery-charge of each terminal along the routing path.
Owner:STRONG FORCE IOT
Circuit for Rendering Energy Storage Devices Parallelable
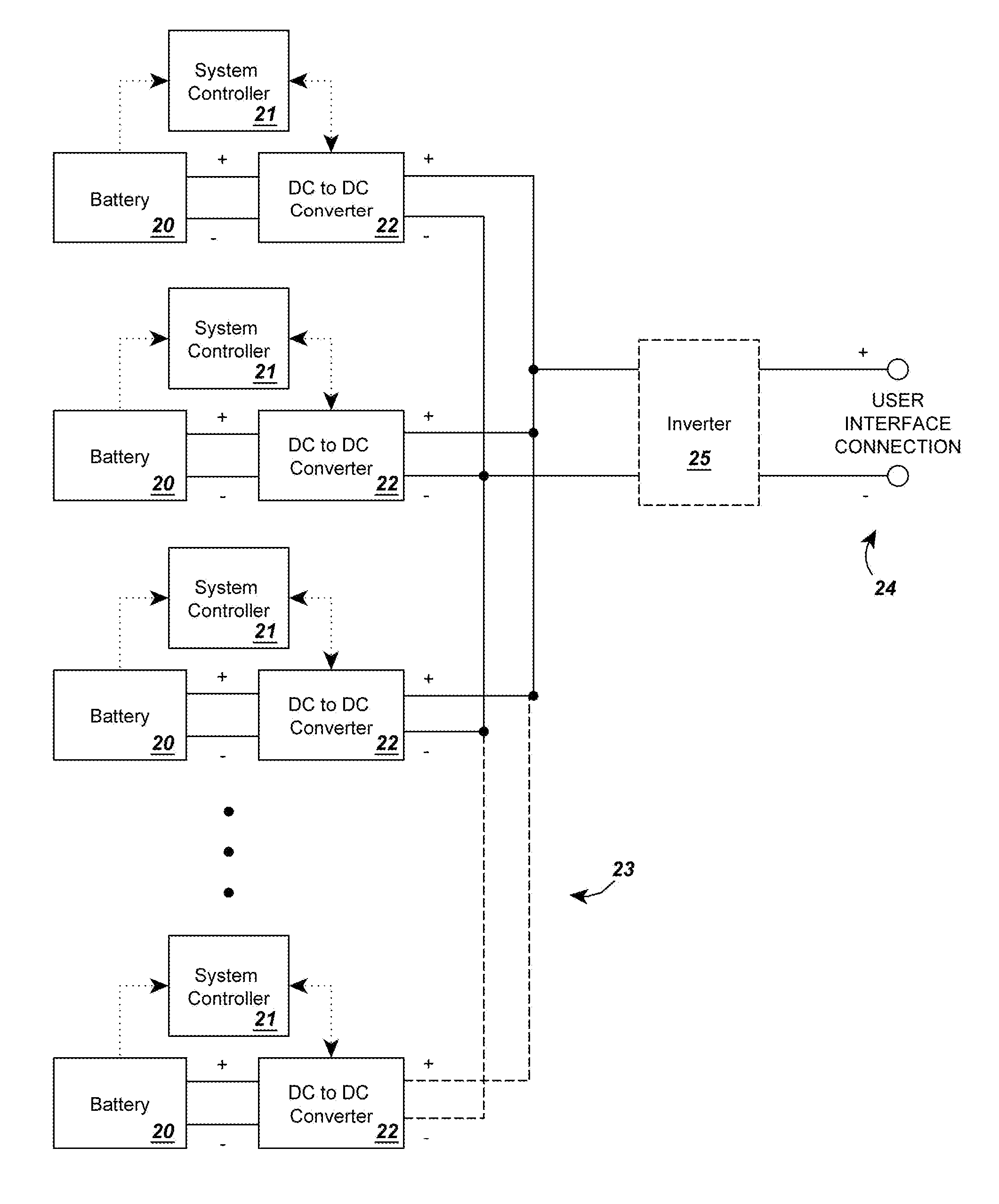
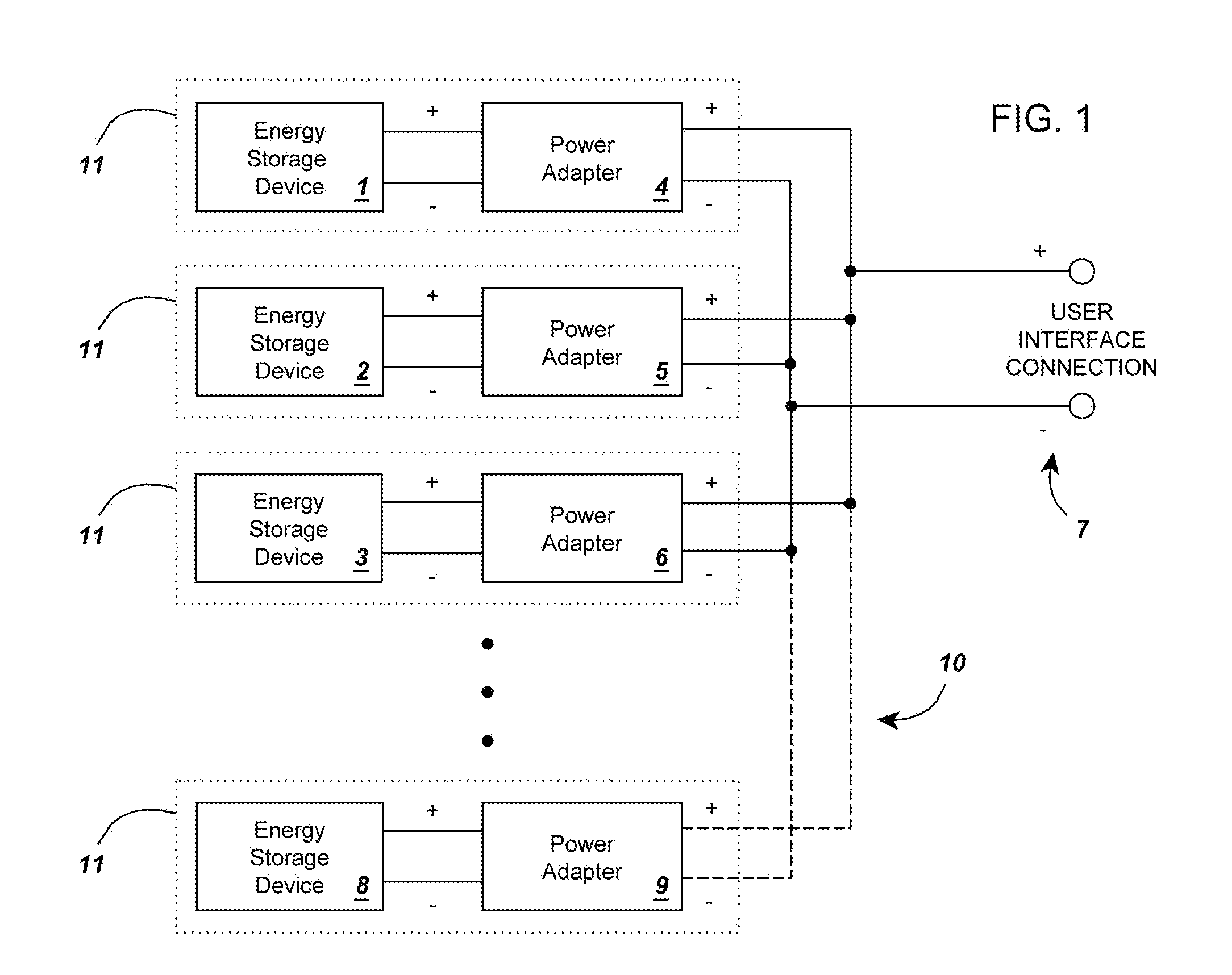
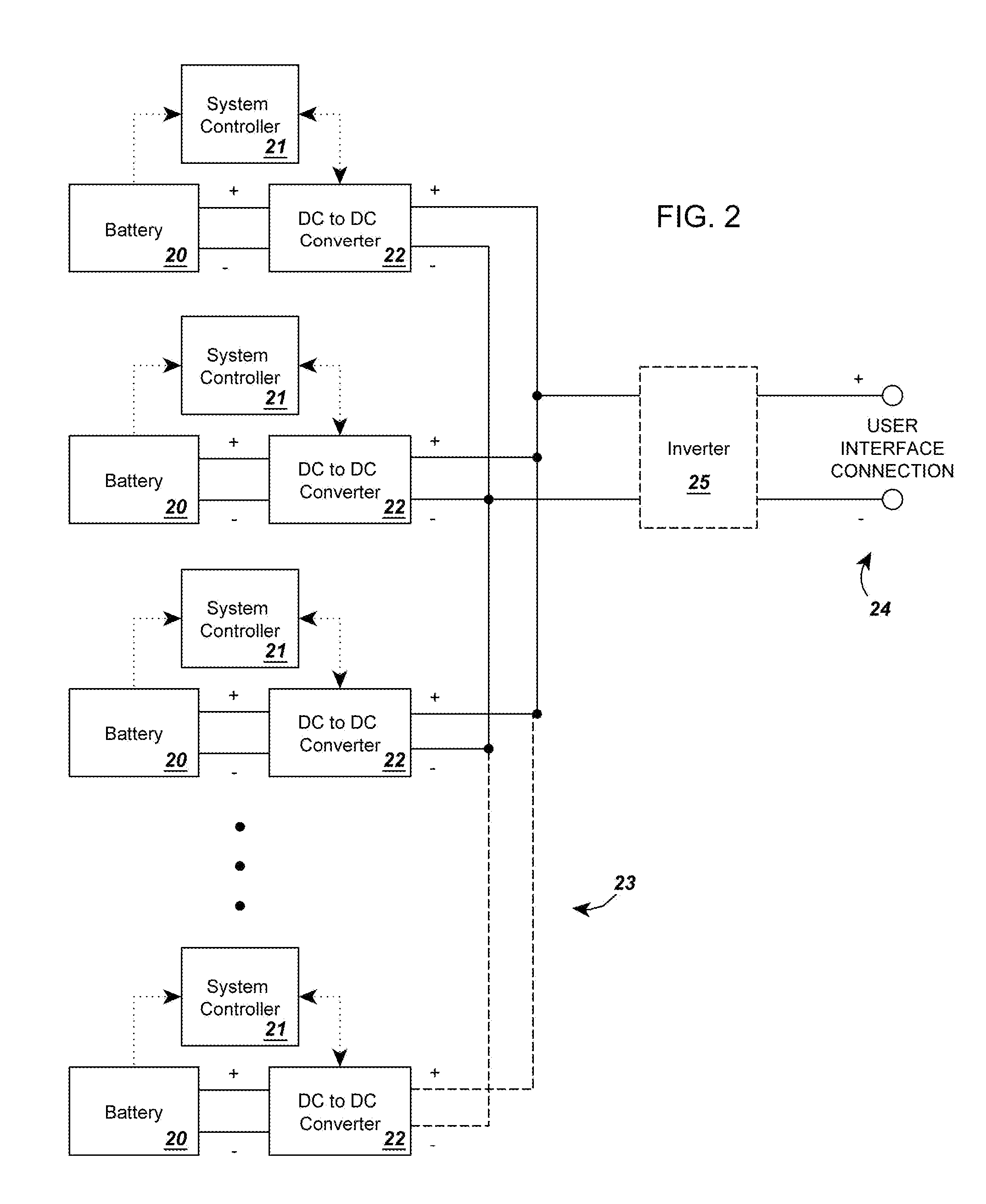
InactiveUS20120274145A1Increase capacityEasy to recycleSecondary cellsDc source parallel operationBattery state of chargeElectrical battery
A circuit for rendering an energy storage device parallelable comprised of an energy storage device connected to a power adapter that converts the potential of the energy storage device into a potential that follows a predetermined function of the state of charge of the energy storage device. When multiple assemblies are paralleled, they may be charged and discharged as a whole with individual storage devices maintaining equal states of charge. The energy storage devices can be batteries with different cell counts, configurations, and energy discharge profiles. In some cases, the power adapters are comprised of DC to DC converters and system controllers that are used to translate each battery's energy discharge profile into a user-determined energy discharge profile that is a predictable function of the battery's state of charge and independent of temperature or other external conditions.
Owner:ENGIE STORAGE SERVICES NA LLC
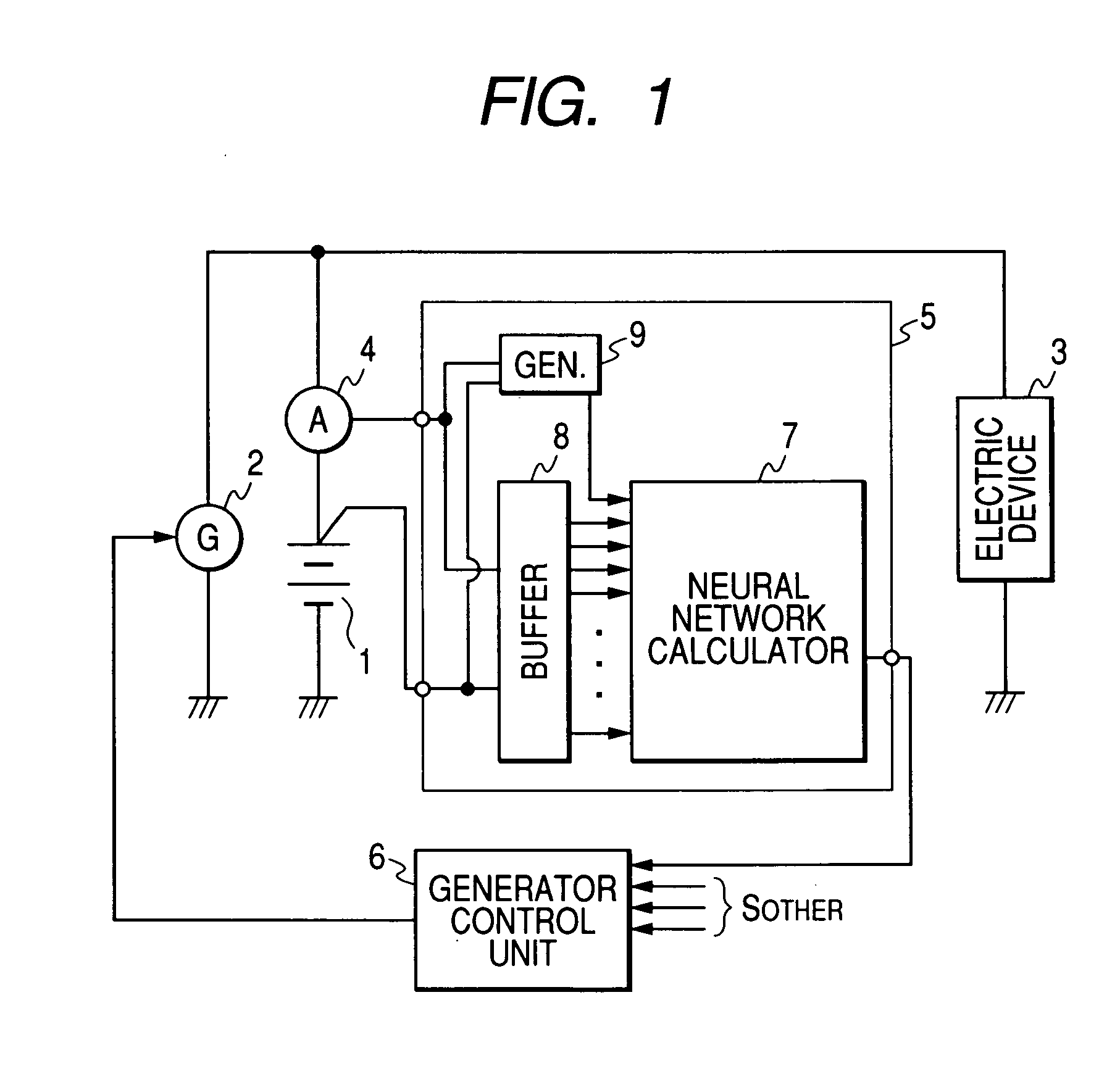
Method and apparatus for detecting charged state of secondary battery based on neural network calculation
InactiveUS20060181245A1Improve accuracyAccurate calculationBatteries circuit arrangementsLighting and heating apparatusBattery state of chargeEngineering
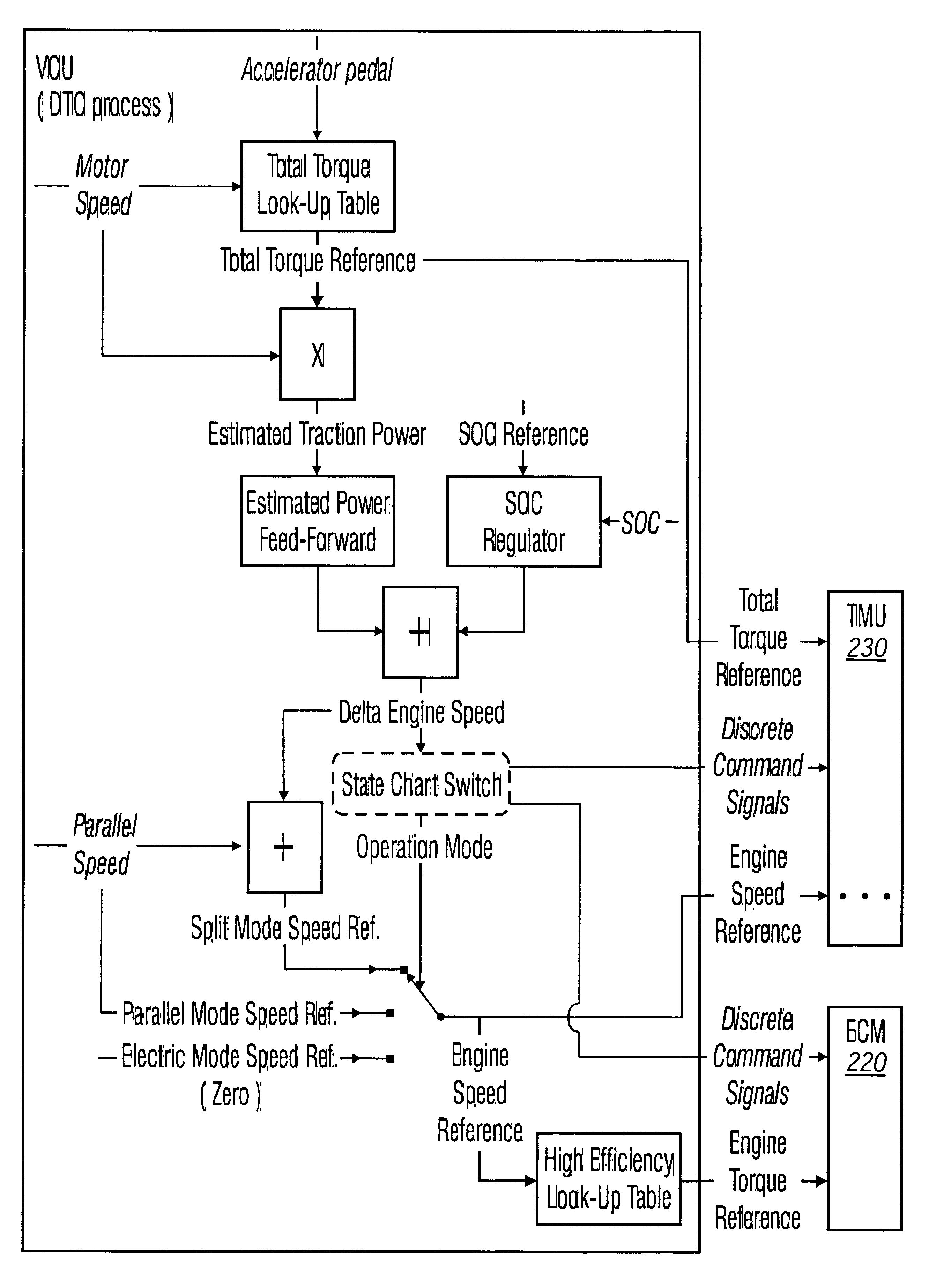
A neural network type of apparatus is provided to detect an internal state of a secondary battery implemented in a battery system. The apparatus comprises a detecting unit, producing unit and estimating unit. The detecting unit detects electric signals indicating an operating state of the battery. The producing unit produces, using the electric signals, an input parameter required for estimating the internal state of the battery. The input parameter reflects calibration of a present charged state of the battery which is attributable to at least one of a present degraded state of the battery and a difference in types of the battery. The estimating unit estimates an output parameter indicating the charged state of the battery by applying the input parameter to neural network calculation.
Owner:DENSO CORP +2
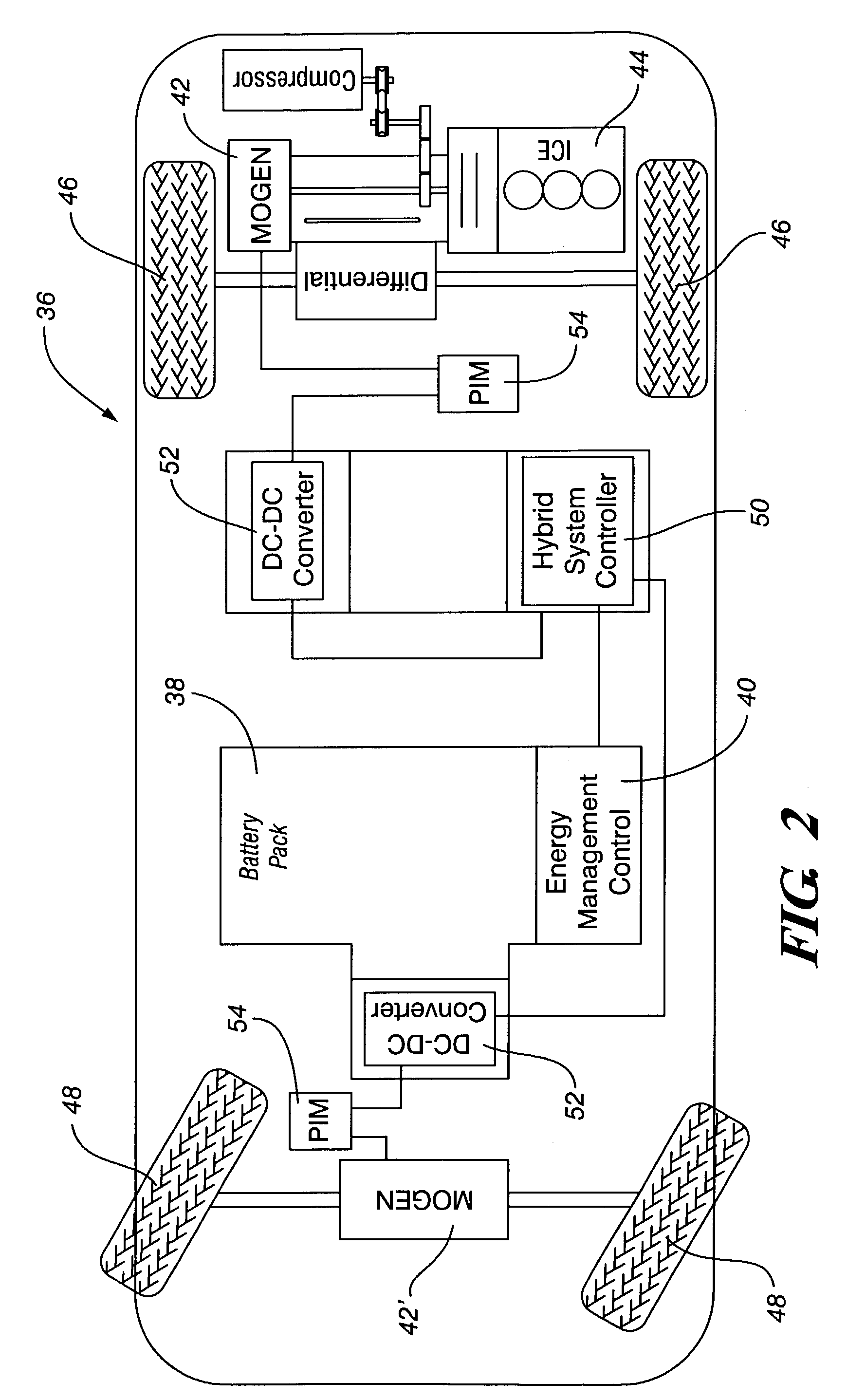
Method and arrangement in a hybrid vehicle for improving battery state-of-charge control and minimizing driver perceptible disturbances
InactiveUS6336063B1Internal combustion piston enginesDigital data processing detailsBattery state of chargeElectric vehicle
Method for minimizing driver perceptible drive train disturbances during take-off in a hybrid electric vehicle when maximized power is often desired is disclosed. The method includes sensing an actual state-of-charge (SOC) value of a battery in a hybrid electric vehicle and a traveling velocity of the vehicle during take-off operation. The sensed actual SOC value is compared with a SOC reference value and computing a delta SOC value as a difference therebetween. A velocity-based SOC calibration factor is looked up that corresponds to the traveling velocity of the vehicle. A combination is utilized of the delta SOC value and the SOC calibration factor as a SOC feedback engine speed control instruction to an engine controller of the hybrid electric vehicle. A driver's desired vehicular acceleration is sensed based on accelerator position. Maximum possible engine power generatable at the sensed vehicle speed is determined, as is a required power value from the power train of the vehicle to meet the driver's desired vehicular acceleration. The maximum possible engine power generatable at the sensed vehicle speed is compared with the required power value and computing a delta power train requirement value as a difference therebetween. A velocity-based and accelerator position-based power calibration factor is looked-up that corresponds to the traveling velocity of the vehicle and the accelerator position. A combination of the delta power train requirement value and the power calibration factor is utilized as a power requirement feed-forward engine speed control instruction to an engine controller of the hybrid electric vehicle.
Owner:VOLOVO CAR CORP
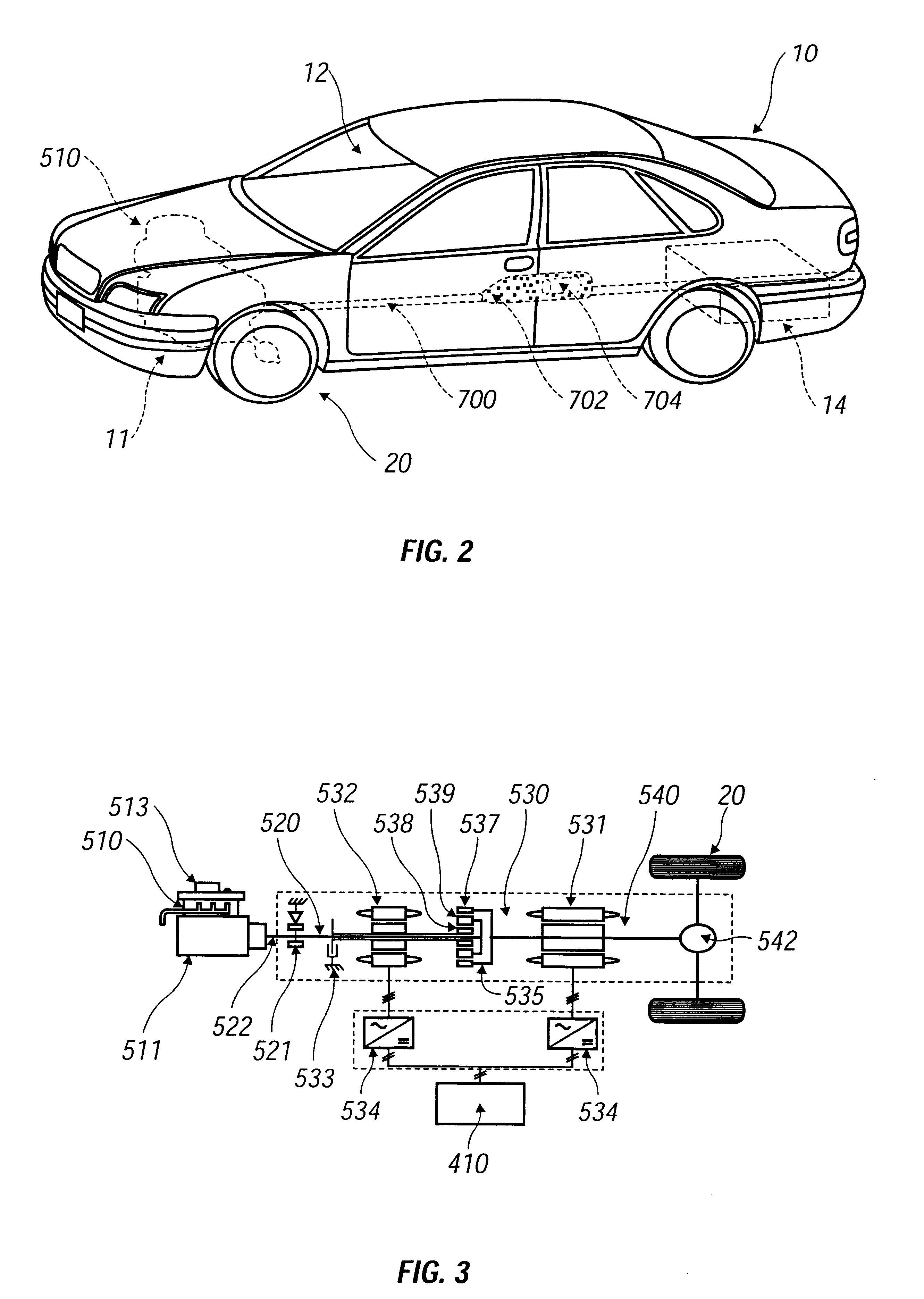
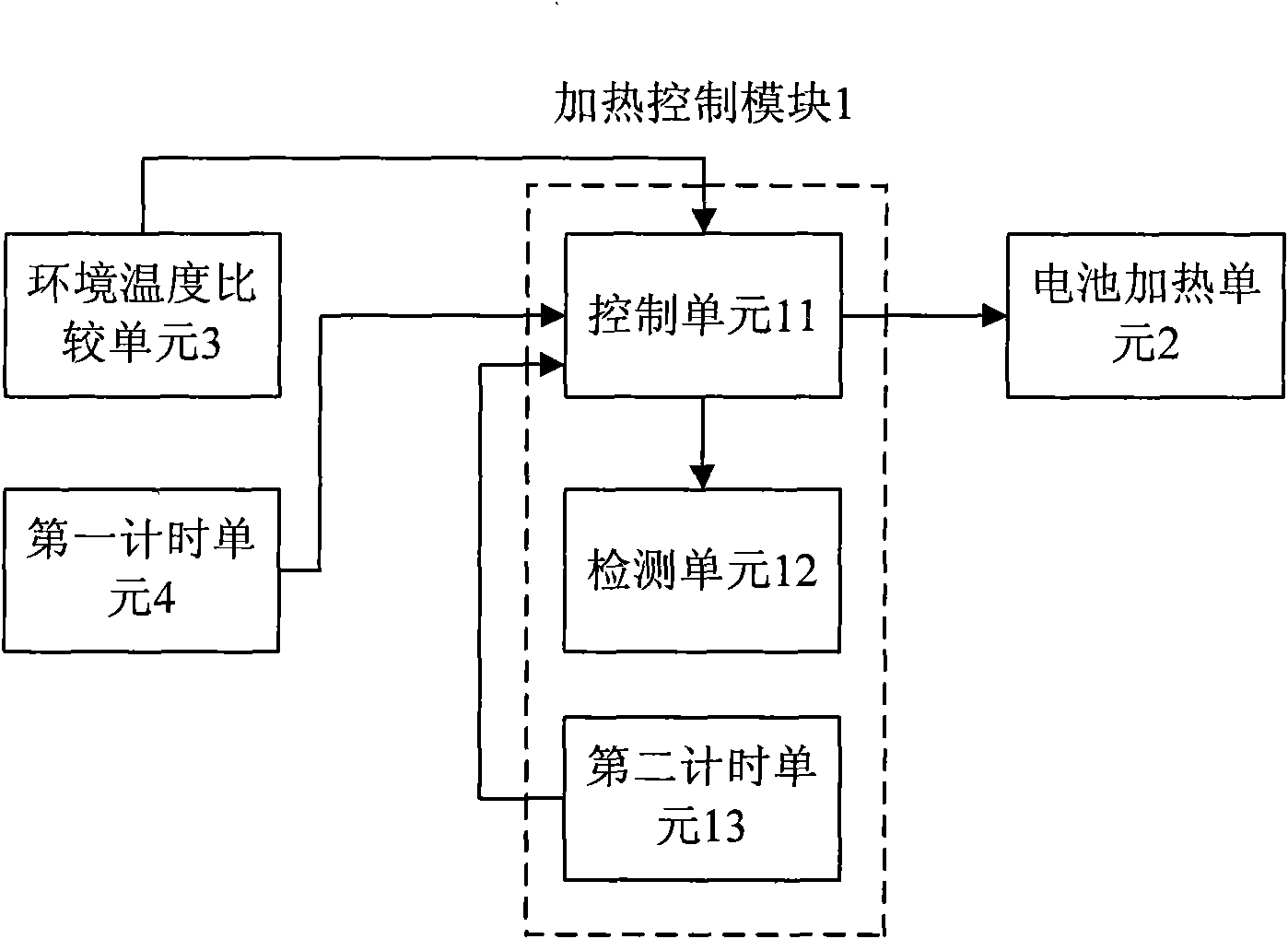
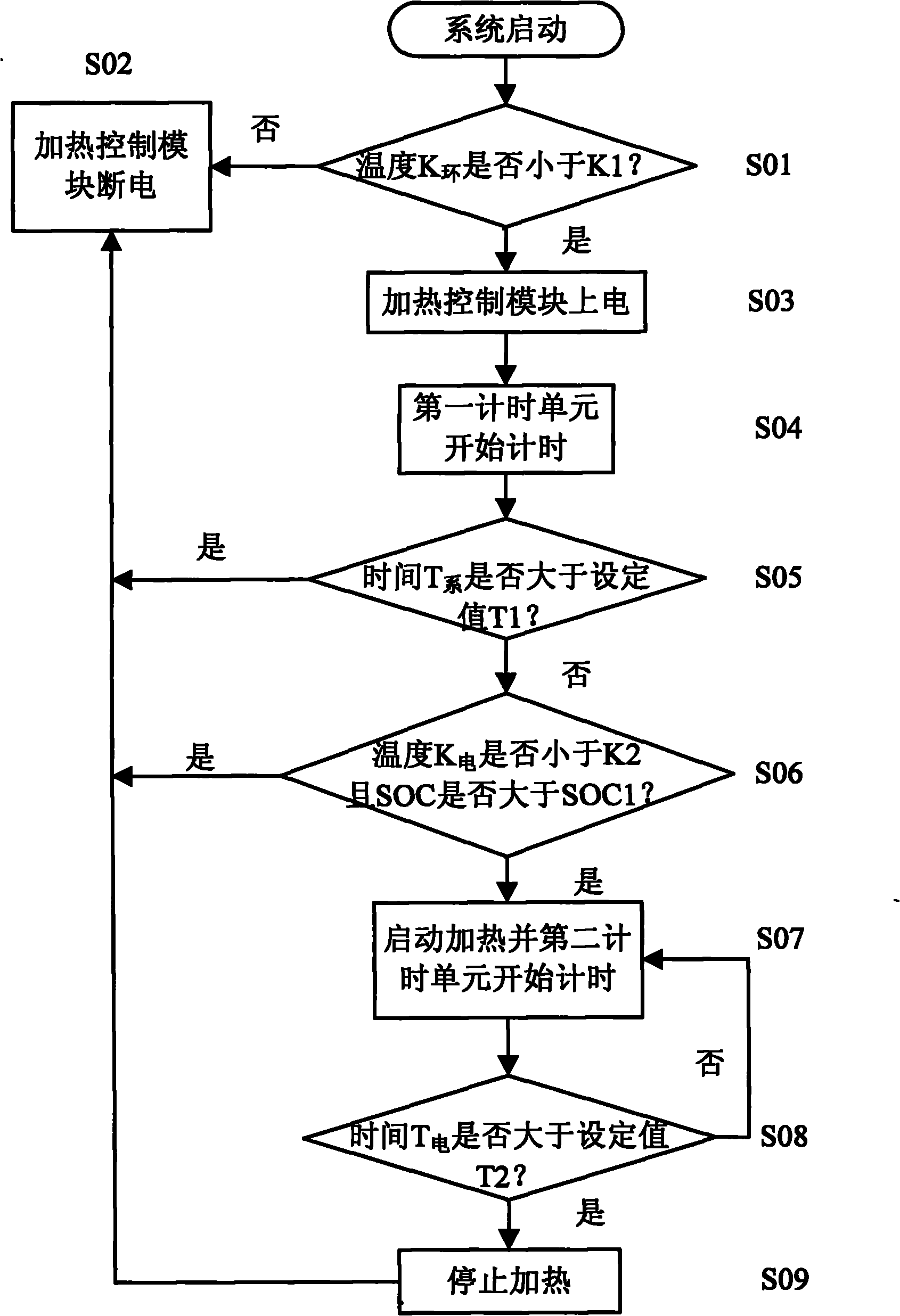
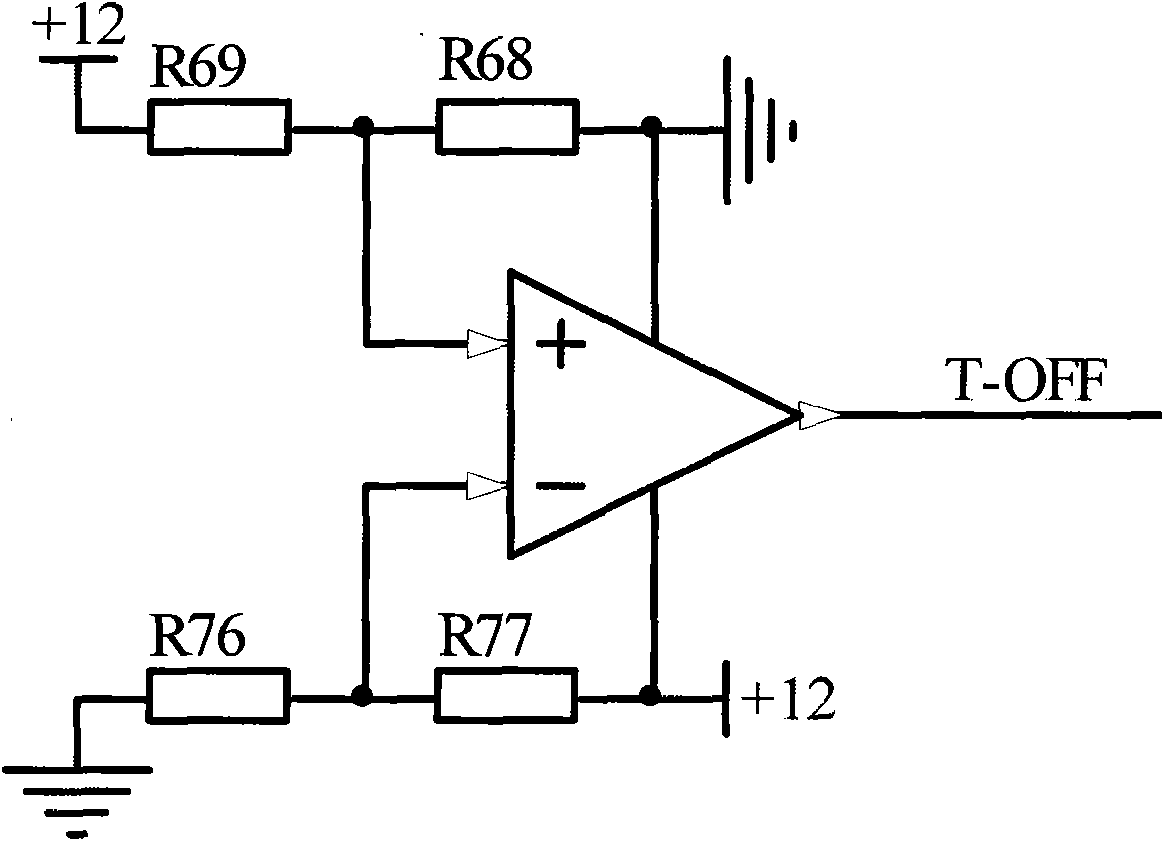
Battery heating control system for vehicles and control method thereof
ActiveCN102055042AGuaranteed charge and discharge performanceWill not harmSecondary cellsBattery state of chargeElectricity
The invention provides a battery heating control system for vehicles and a control method thereof. The battery heating control system comprises a heating control module and a battery heating unit; the heating control module is electrically connected with the battery heating unit; the battery heating unit is used for heating a battery; the heating control module is used for starting the heating ofthe battery by the battery heating unit when the condition of heating starting is met and stopping the heating of the battery by the battery heating unit when the condition of heating stop is met, wherein the starting of the battery heating unit meets the following conditions simultaneously that: the ambient temperature K ambient of the system reaches a temperature set value K1; the battery temperature K battery reaches a temperature set value K2; and the battery state of charge (SOC) reaches a set value SOC1 of the battery SOC. The battery heating control system for the vehicles and the control method can better protect the battery.
Owner:BYD CO LTD
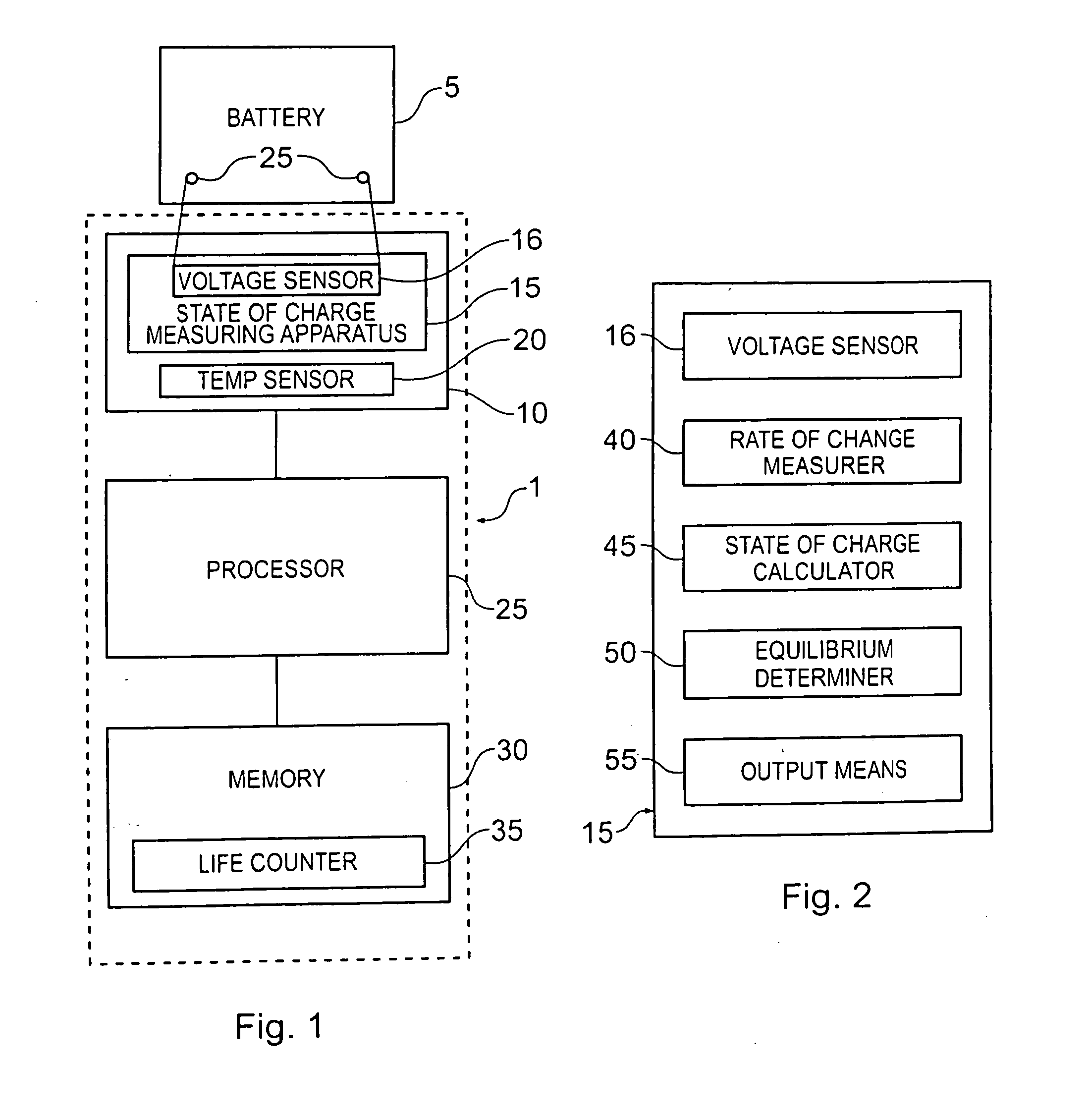
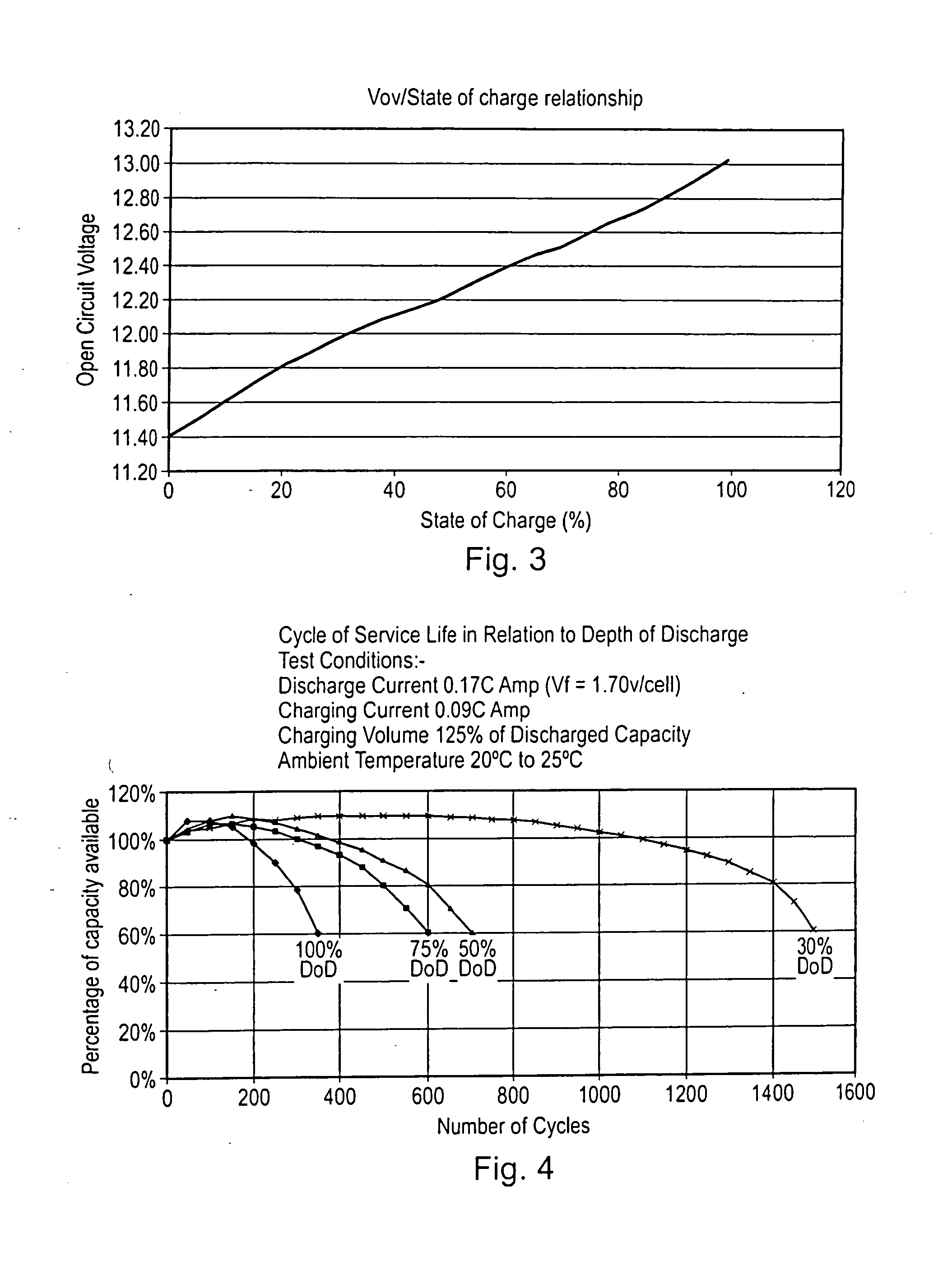
Battery life monitor and battery state of charge monitor
InactiveUS20050017685A1Accurate readingBatteries circuit arrangementsElectrical testingBattery state of chargeEngineering
The electrolyte inside a lead and acid battery is inaccessible and measurements of the voltage across a battery's terminals do not necessarily give a good indication of the future performance of a battery. Furthermore, although the state of charge of a battery can be determined from measurement of the voltage across its terminals, these measurements will be misleading unless they are taken when the battery is in equilibrium. Accordingly, the present invention proposes a battery monitor which takes measurements from a battery and is configured to detect one or more types of event affecting the battery life and to subtract a predetermined amount from a life counter representing the remaining available life of the battery, each time such an event is detected. The monitor is also configured to calculate the state of charge of the battery, but only when the battery is in equilibrium.
Owner:YUASA BATTERY UK
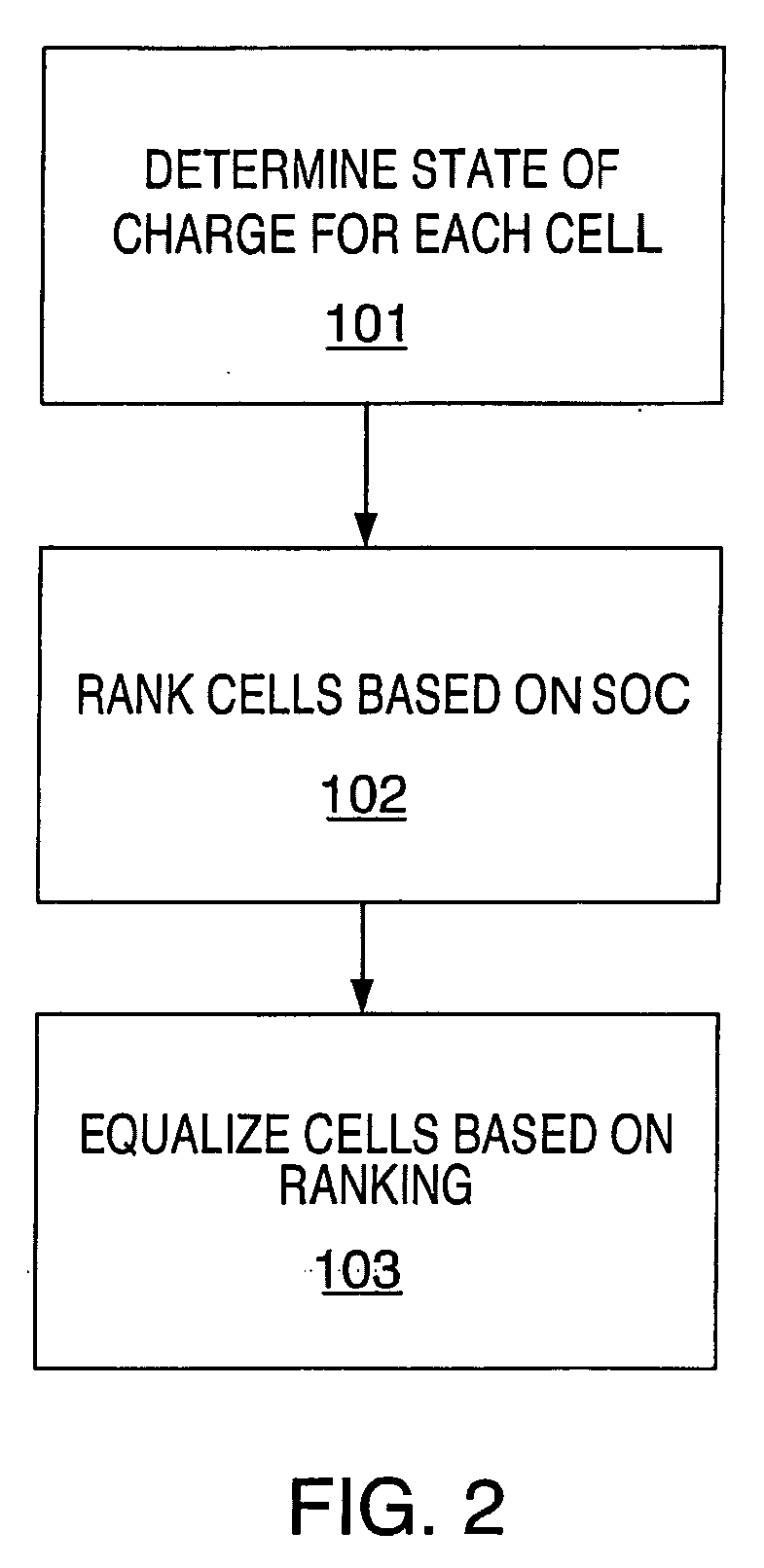
Method and system for cell equalization using state of charge
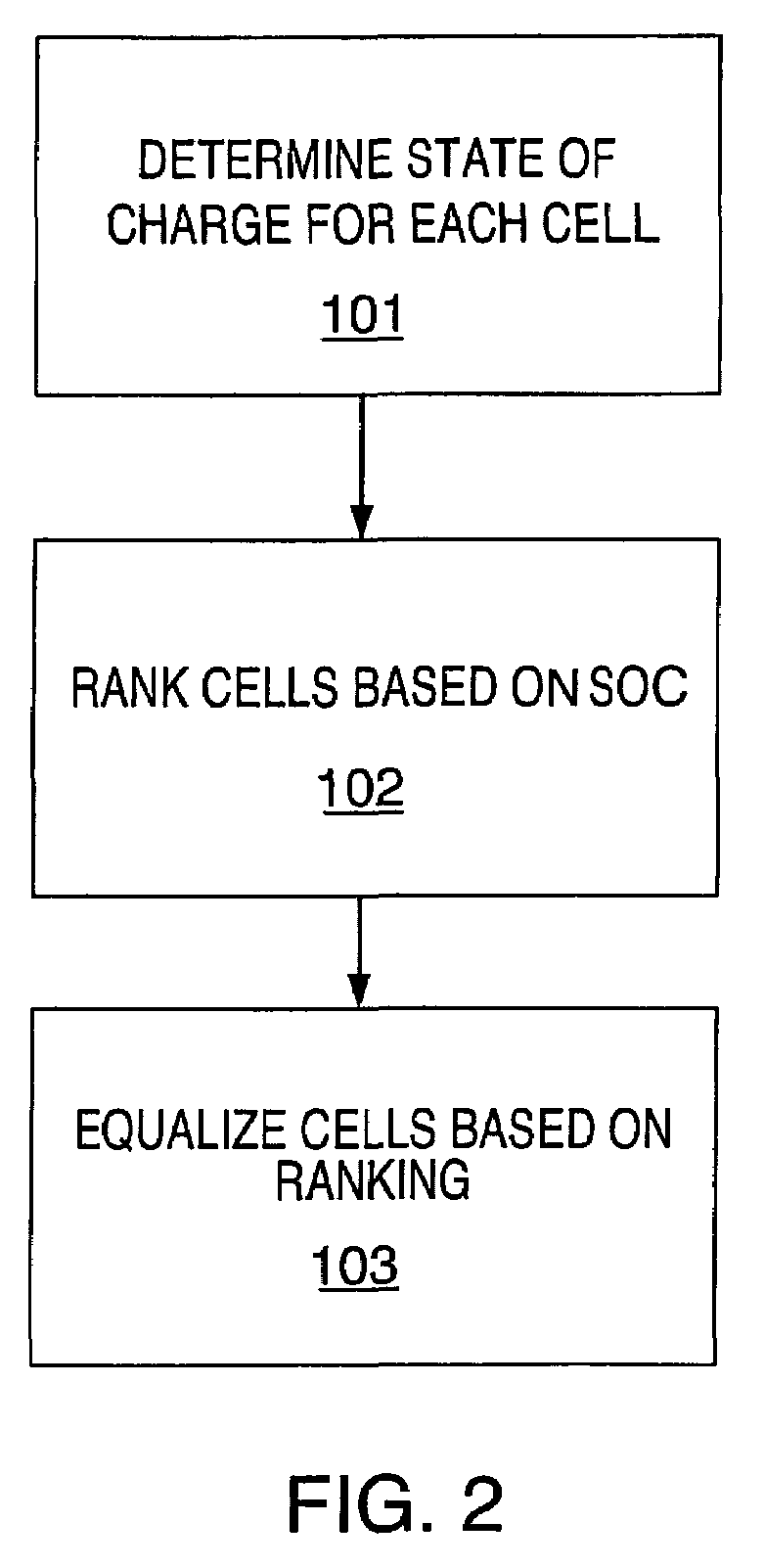
ActiveUS20060097698A1Easy to determineCharge equalisation circuitCircuit monitoring/indicationKaiman filterBattery state of charge
Owner:LG ENERGY SOLUTION LTD
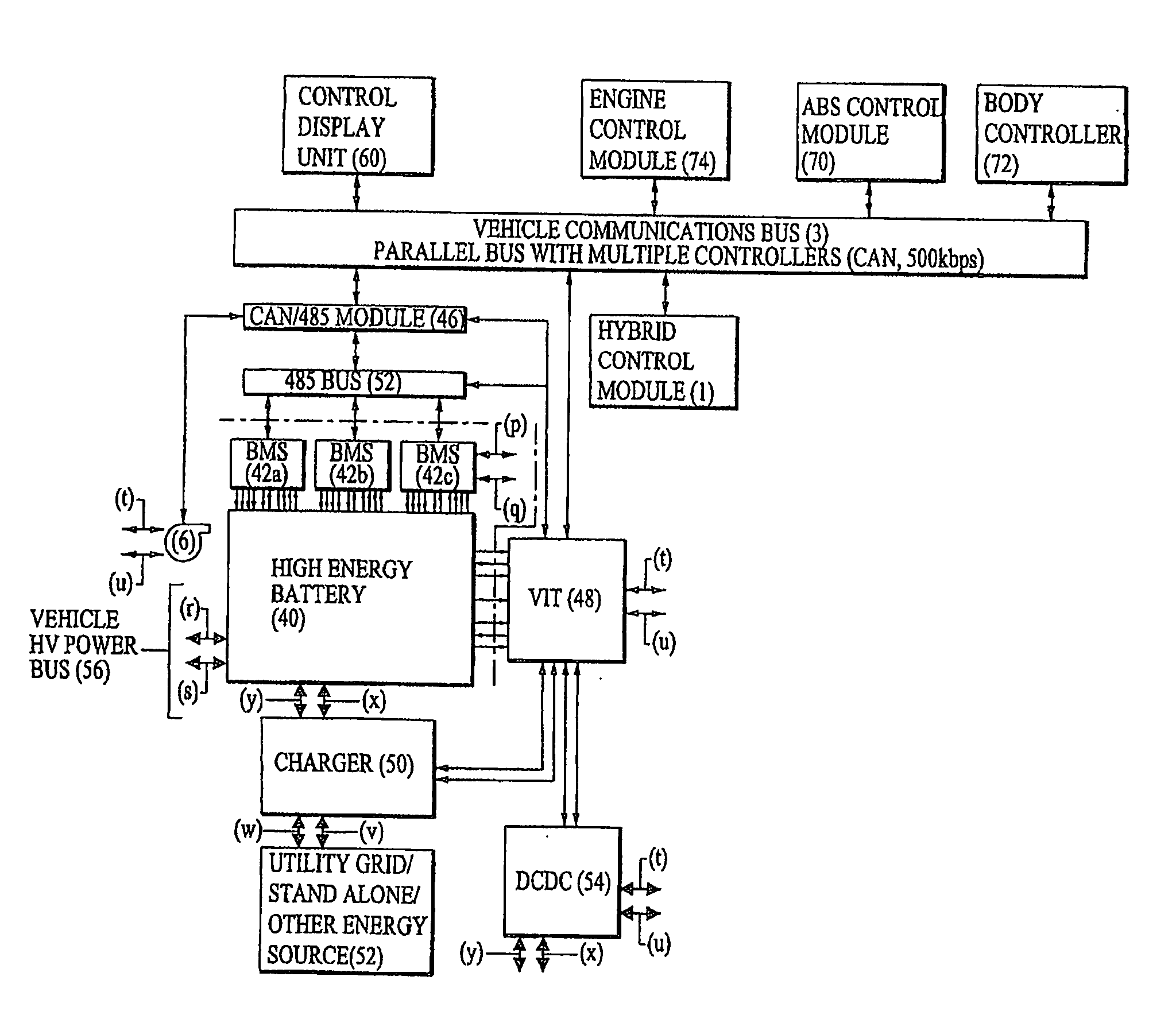
Method And System For Retrofitting A Full Hybrid To Be A Plug-In Hybrid
InactiveUS20100106351A1Limit its operationEasy to manageCircuit monitoring/indicationDigital data processing detailsBattery state of chargeBattery charge
A retrofit system for converting a hybrid vehicle to a plug-in hybrid comprises a battery for storing a charge sufficient to provide a vehicle with an electric-only driving range in excess of 5 miles, a battery management system for monitoring battery parameters indicative of the state of battery charge to produce a signal indicative thereof; and a control unit responsive the battery management system to report an inflated state of battery charge to the hybrid vehicle's hybrid control system to maintain the gasoline engine in a deactivated condition over an extended driving range.
Owner:EXERGONIX INC
Battery capacity measuring and remaining capacity calculating system
InactiveUS6621250B1Quick calculationEnhanced advantageCircuit monitoring/indicationInternal combustion piston enginesBattery state of chargeIntegrator
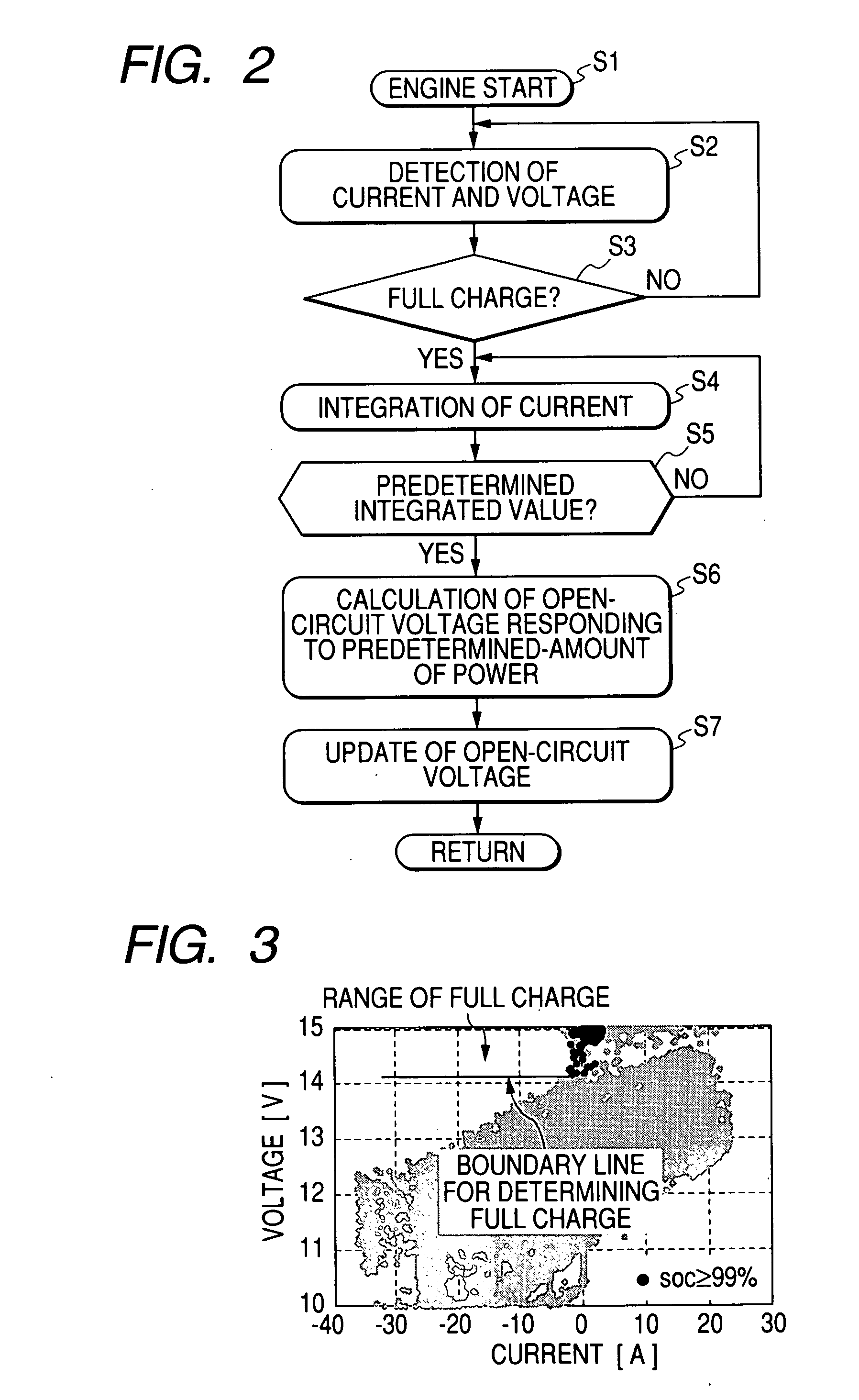
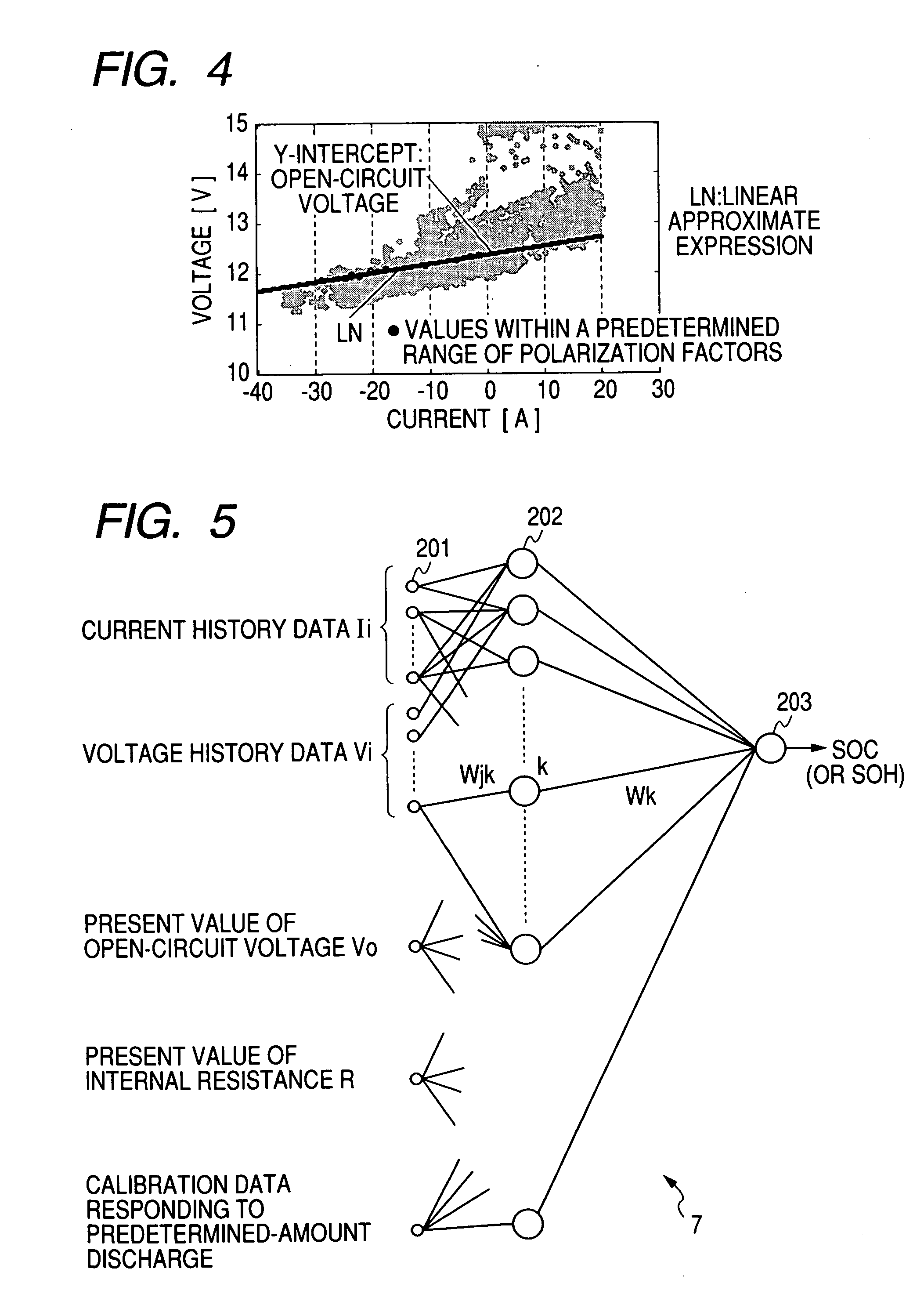
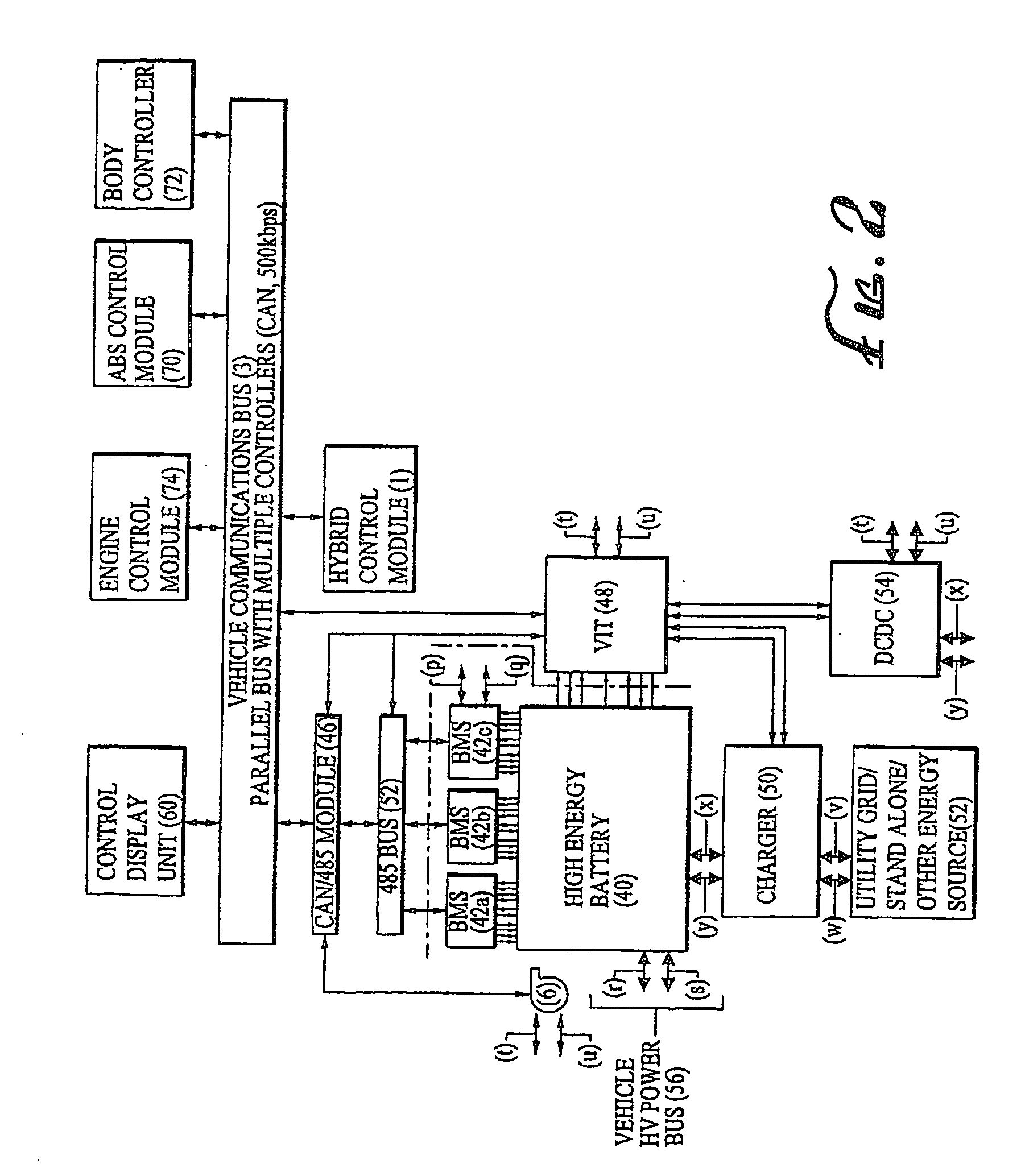
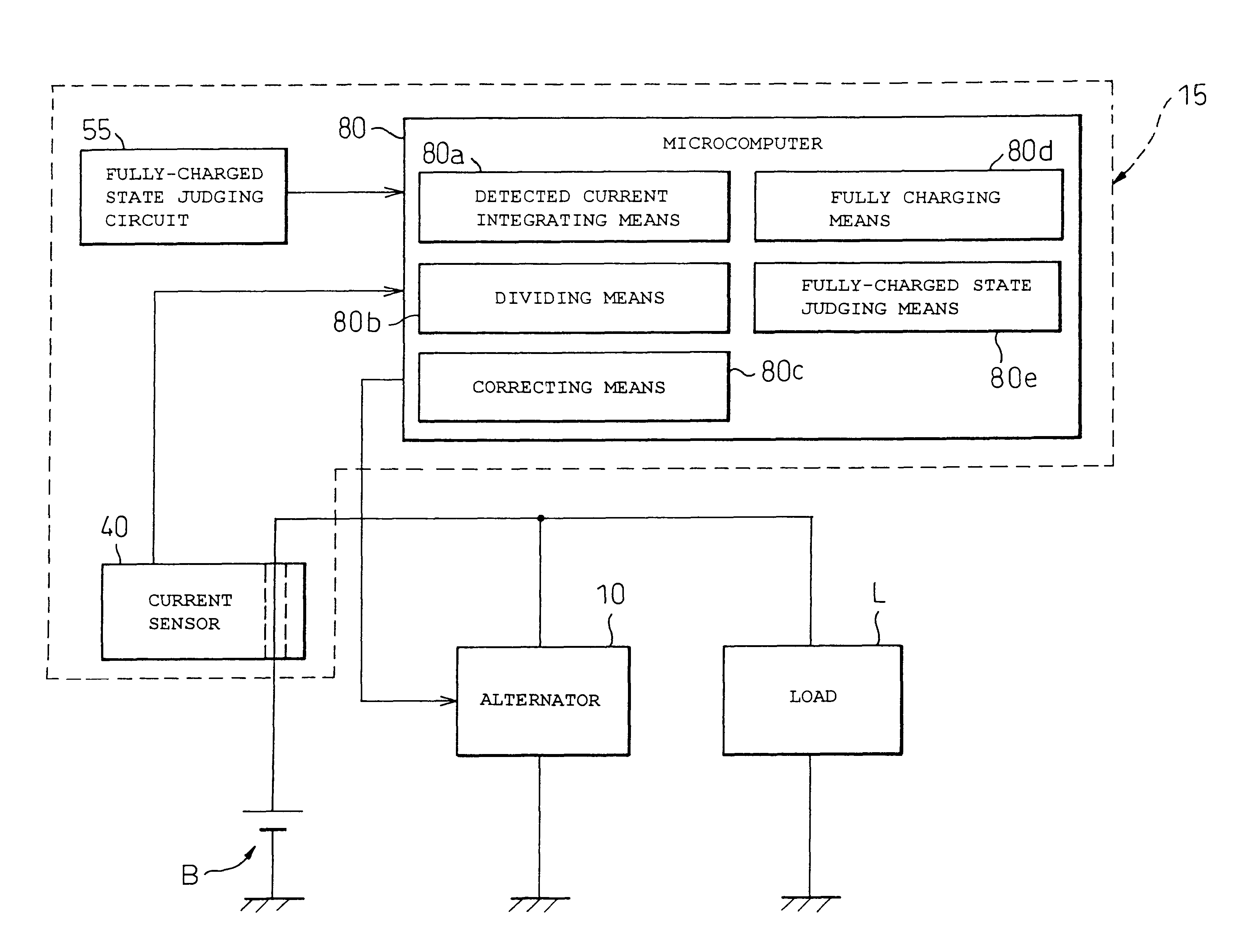
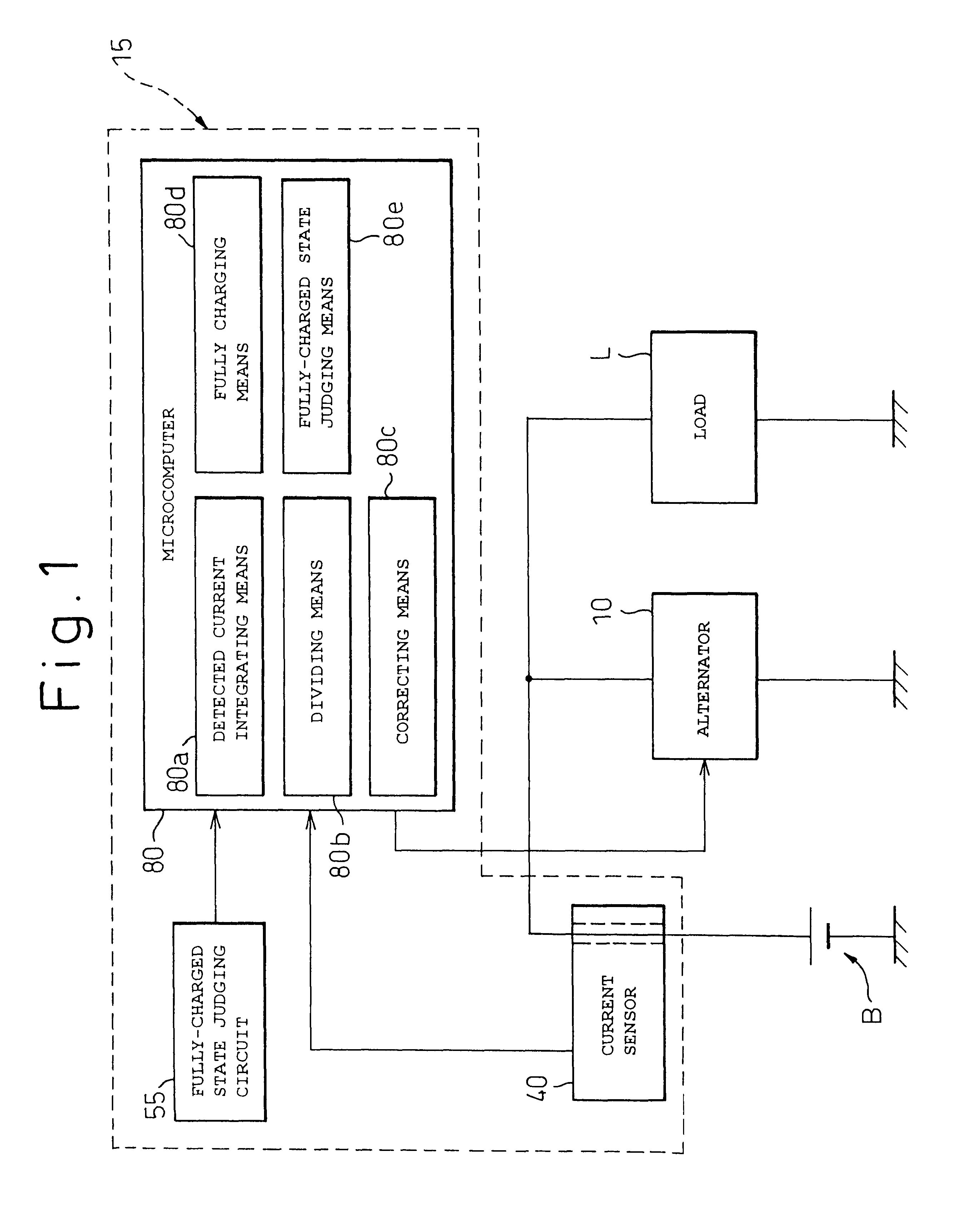
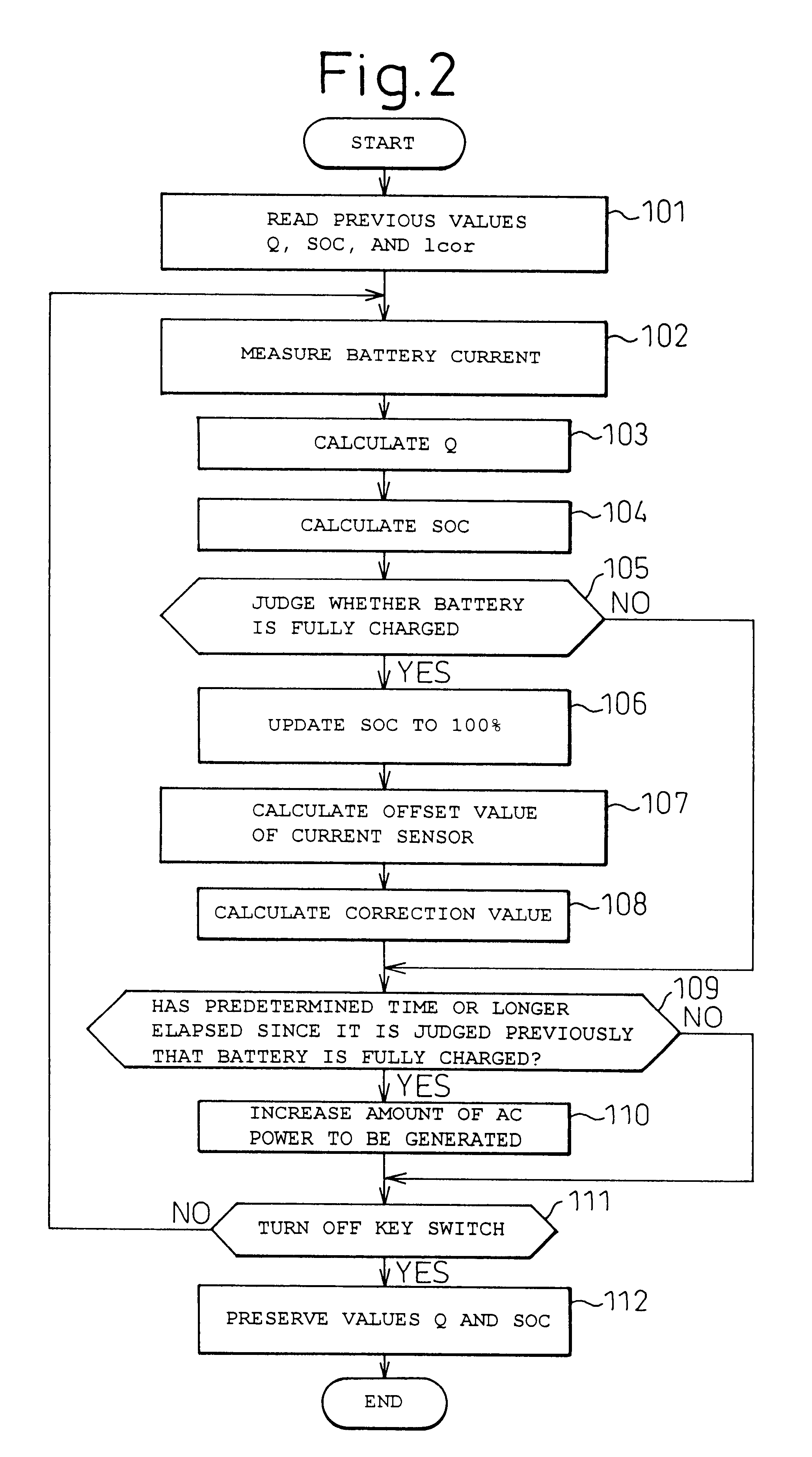
A battery capacity measuring device in accordance with the present invention has a fully-charged state detector (80e), a detected current integrator (80a), a divider (80b), and a corrector (80c) incorporated in a microcomputer (80). The fully-charged state detector detects that a battery is fully charged. The detected current integrator integrates current values that are detected by a current sensor during a period from the instant the battery is fully charged to the instant it is fully charged next. The divider divides the integrated value of detected current values by the length of the period. The corrector corrects a detected current using the quotient provided by the divider as an offset. Furthermore, a remaining battery capacity calculating system comprises a voltage detecting unit (50), a current detecting unit (40), an index calculating unit, a control unit, and a calculating unit. The voltage detecting unit detects the voltage at the terminals of a battery. The current detecting unit detects a current flowing through the battery. The index calculating unit calculates the index of polarization in the battery according to the detected current. The control unit controls the output voltage of an alternator so that the index of polarization will remain within a predetermined range which permits limitation of the effect of polarization on the charged state of the battery. When the index of polarization remains within the predetermined range, the calculating unit calculates the remaining capacity of the battery according to the terminal voltage of the battery, that is, the open-circuit voltage of the battery.
Owner:TOYOTA JIDOSHA KK +1
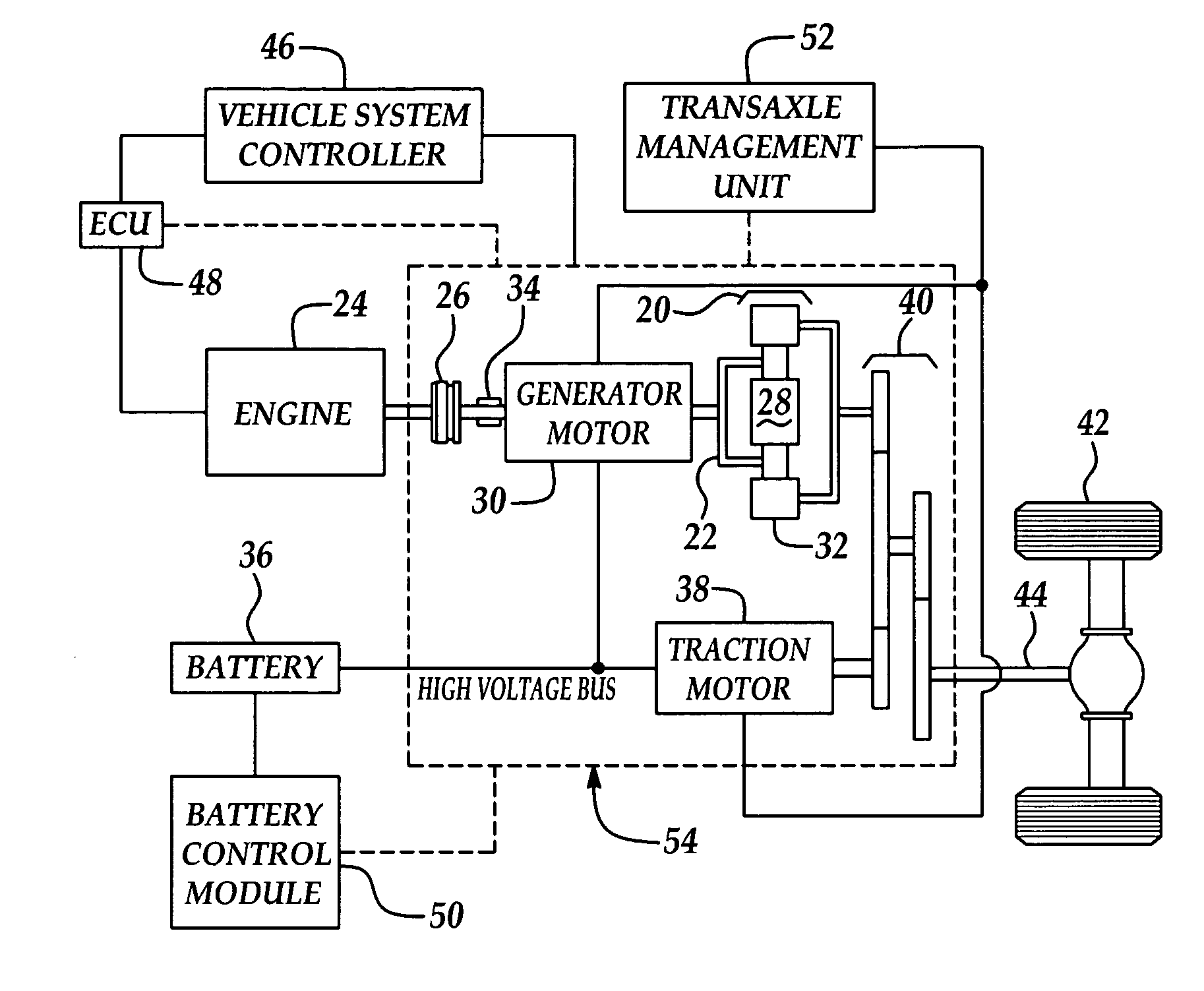
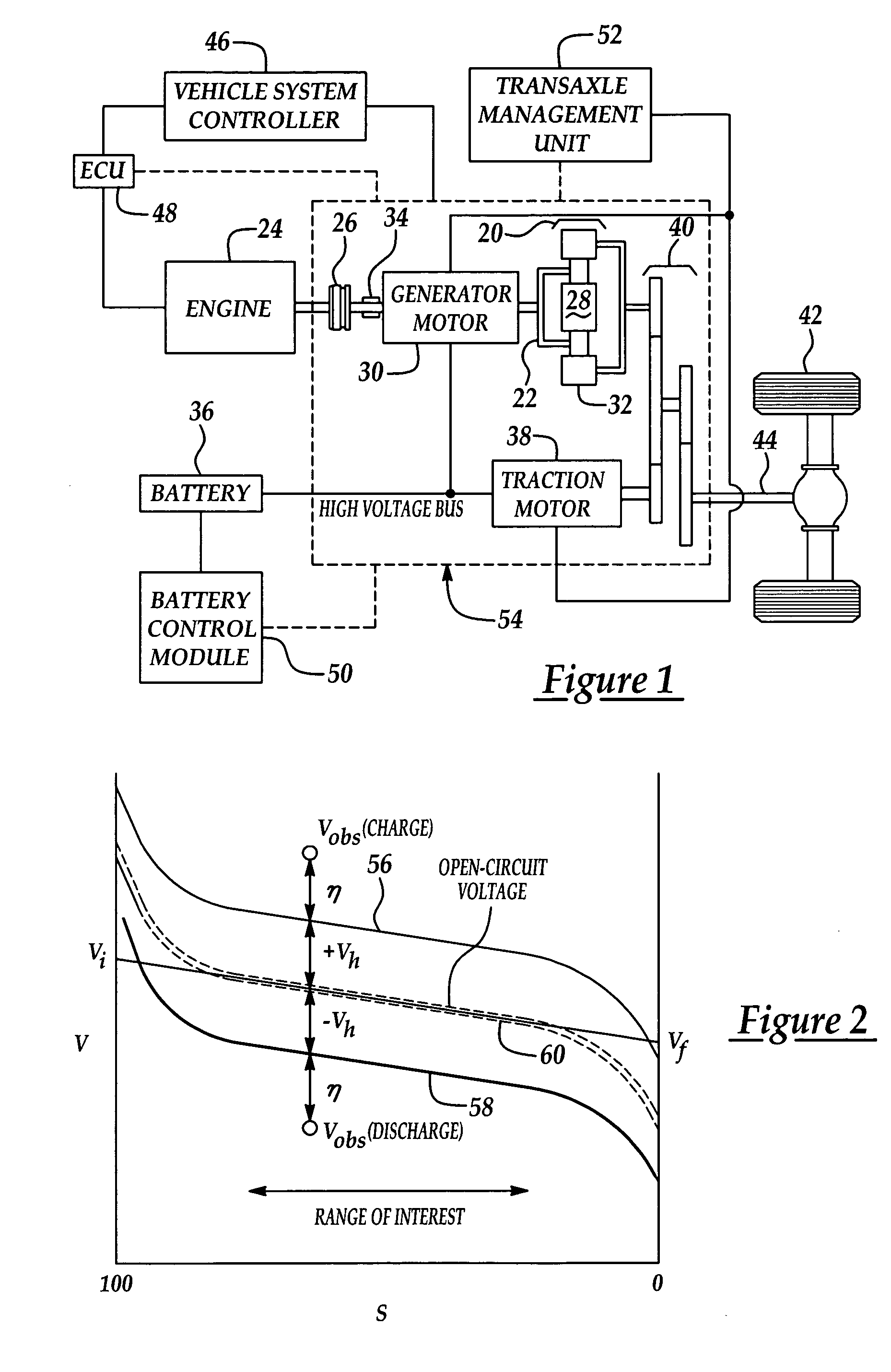
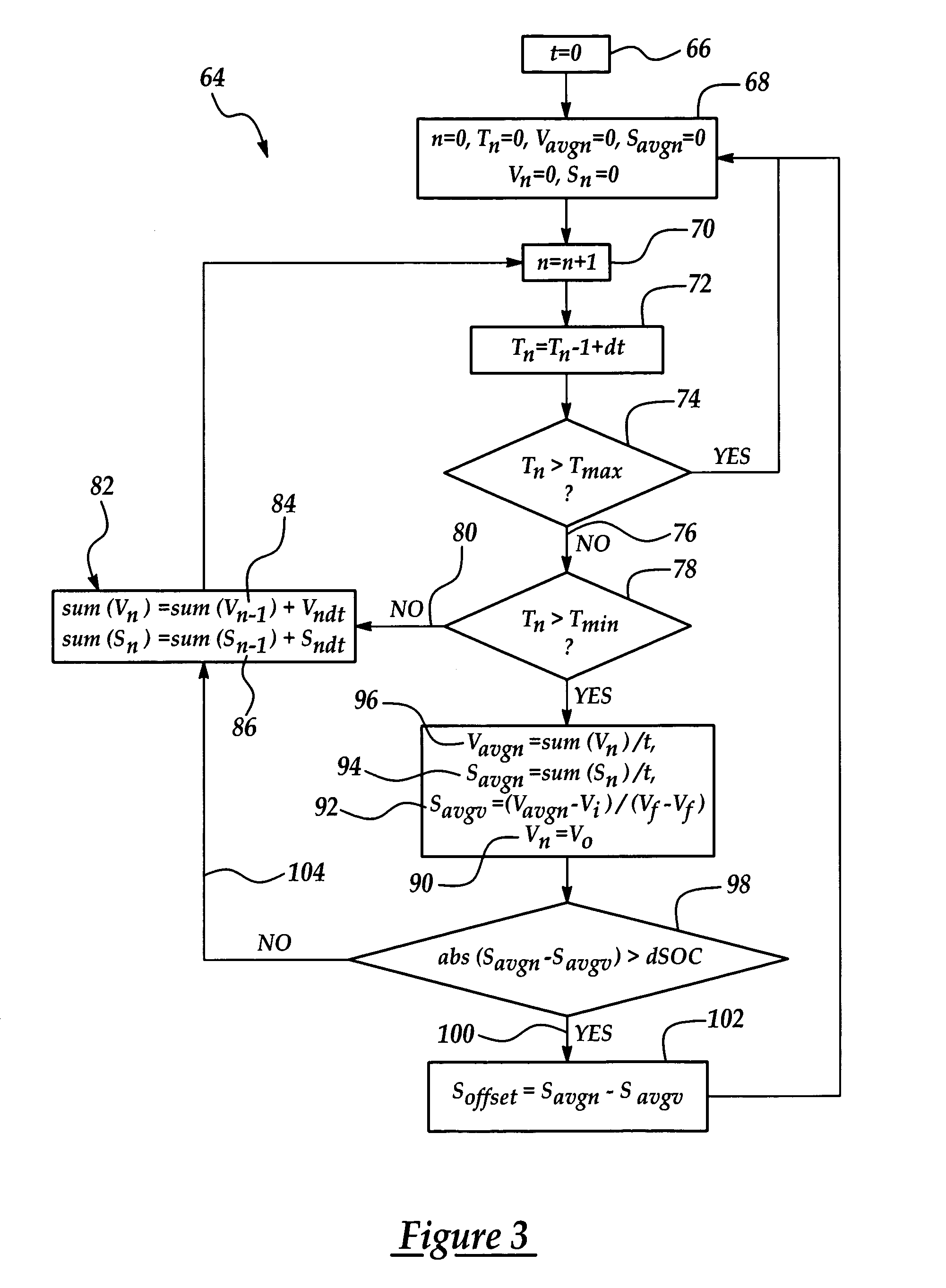
Calculation of state of charge offset using a closed integral method
InactiveUS7233128B2Eliminate hysteresisCircuit monitoring/indicationEmergency protective circuit arrangementsHysteresisBattery state of charge
Owner:FORD GLOBAL TECH LLC
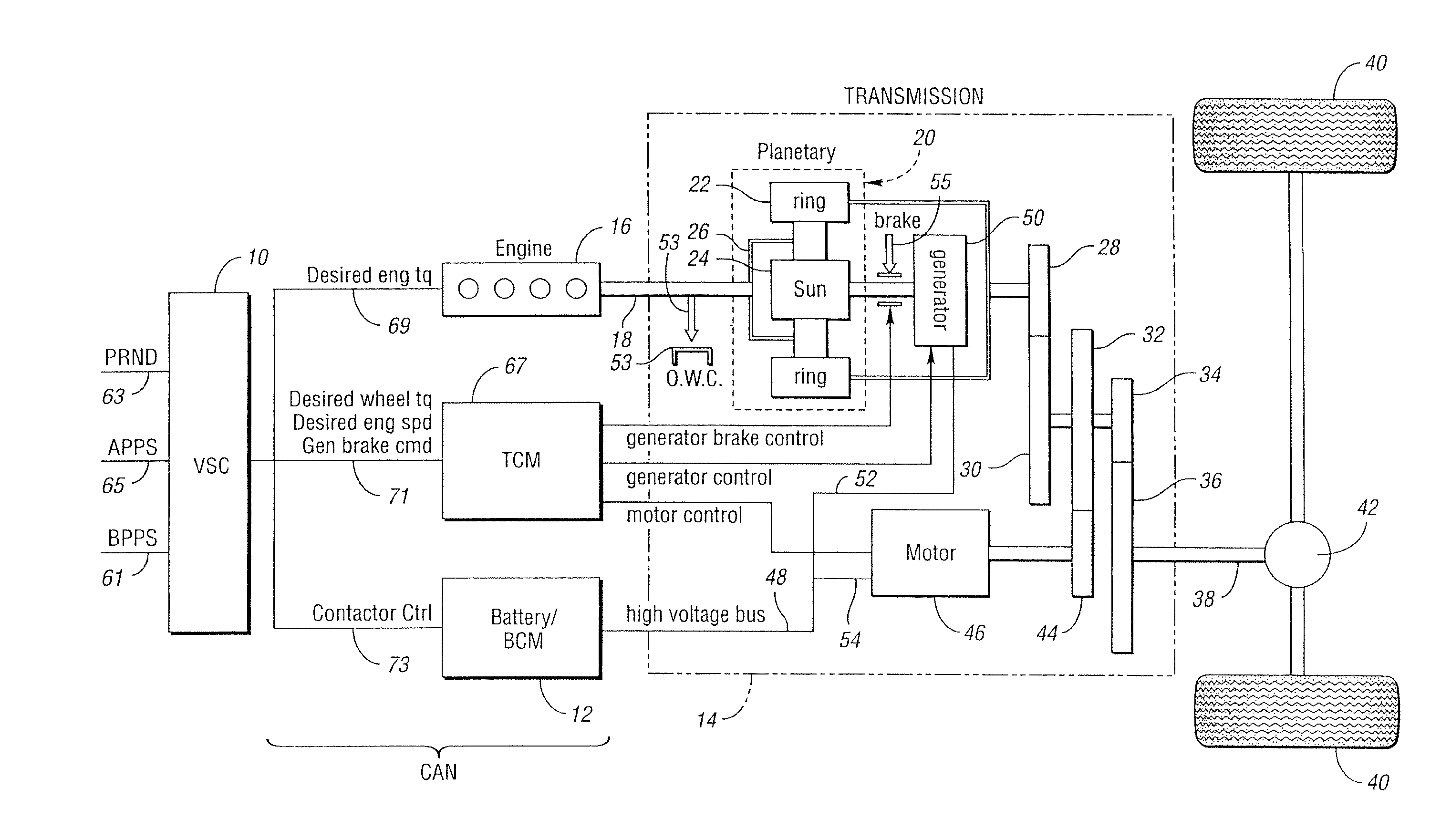
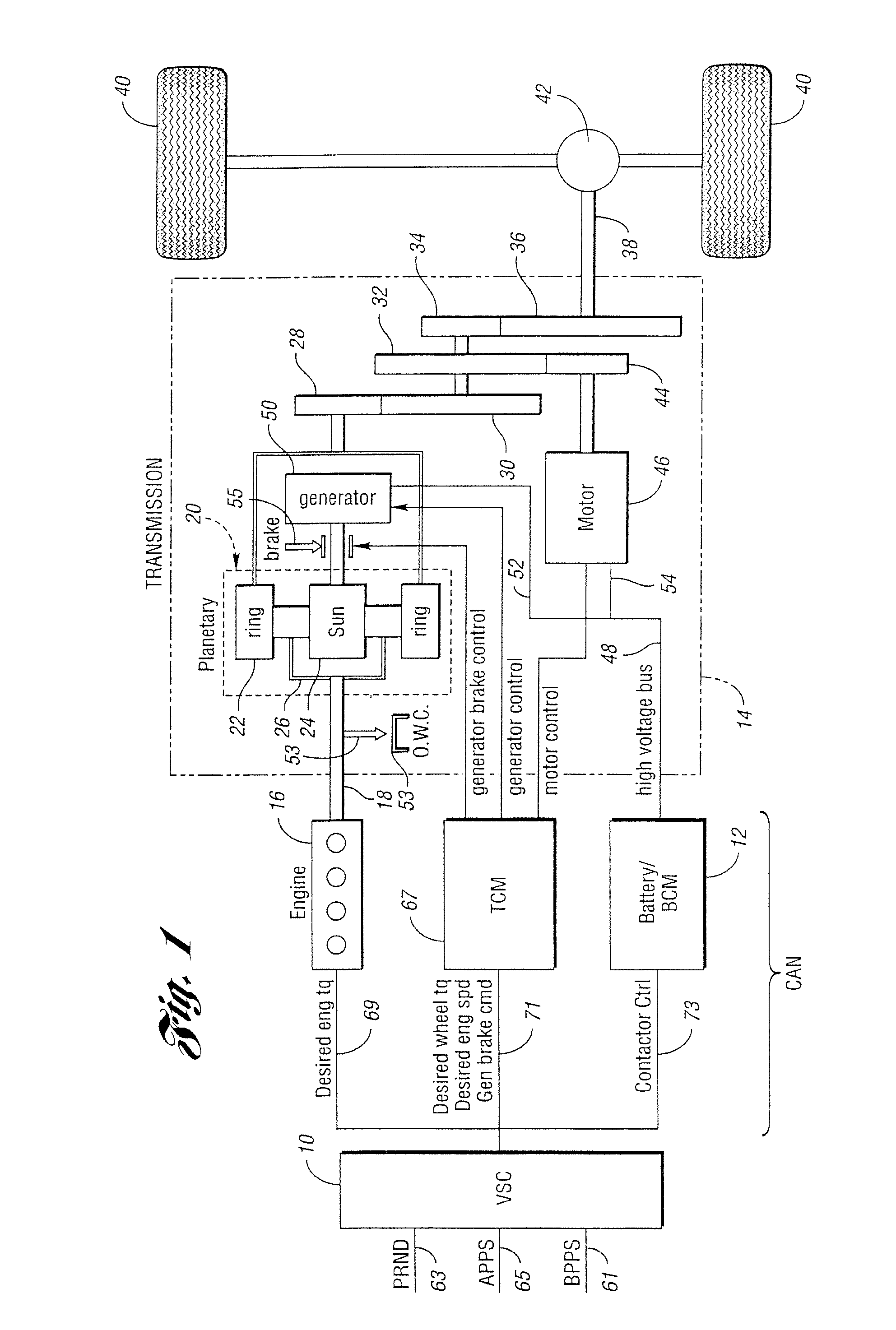
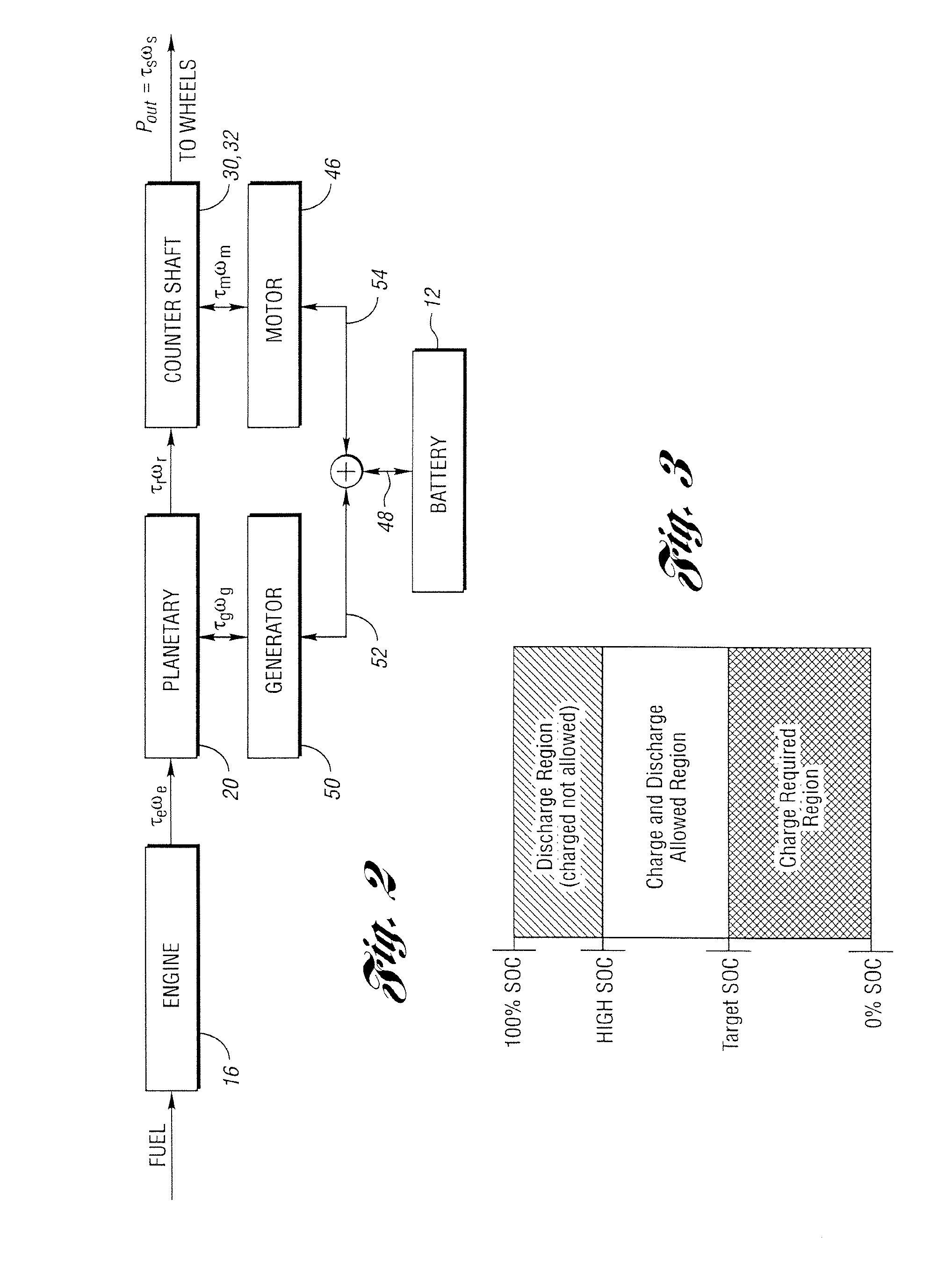
Controller and control method for a hybrid electric vehicle powertrain
ActiveUS6994360B2Battery is prevented from overchargingHybrid vehiclesElectric propulsion mountingBattery state of chargePower mode
A parallel hybrid electric vehicle powertrain and control method wherein an internal combustion engine and an electric motor system define a split power operating mode, a generator drive mode and a parallel power mode. Provision is made, when the motor is inoperable, for preventing discharge of a battery in the electric motor system when the battery state-of-charge is low and for delivering power from the battery when the battery state-of-charge is greater than a predetermined value.
Owner:FORD GLOBAL TECH LLC
Rechargeable hearing aid
ActiveUS20070104343A1Enhanced couplingDeaf aid adaptationSets using external connectionBattery state of chargeCoupling
A hearing aid having a rechargeable battery and a charger for charging the battery include means for transferring data means for transferring data unidirectionally or bidirectionally between the hearing aid and the charger using either a magnetic field, light, or sound. The hearing aid includes means for detecting the charge state of a battery and for sending a signal to the charger indicative of the charge state. For acoustic coupling, the charger includes a microphone and a speaker and also includes a chamber for receiving at least a portion of the hearing aid.
Owner:ZOUNDS LLC FORMERLY ZOUNDS ACQUISITION
Battery charger apparatus
InactiveUS6515456B1Improve economyProlong lifeElectric powerCharge maintainance charging/dischargingBattery state of chargeAlternator
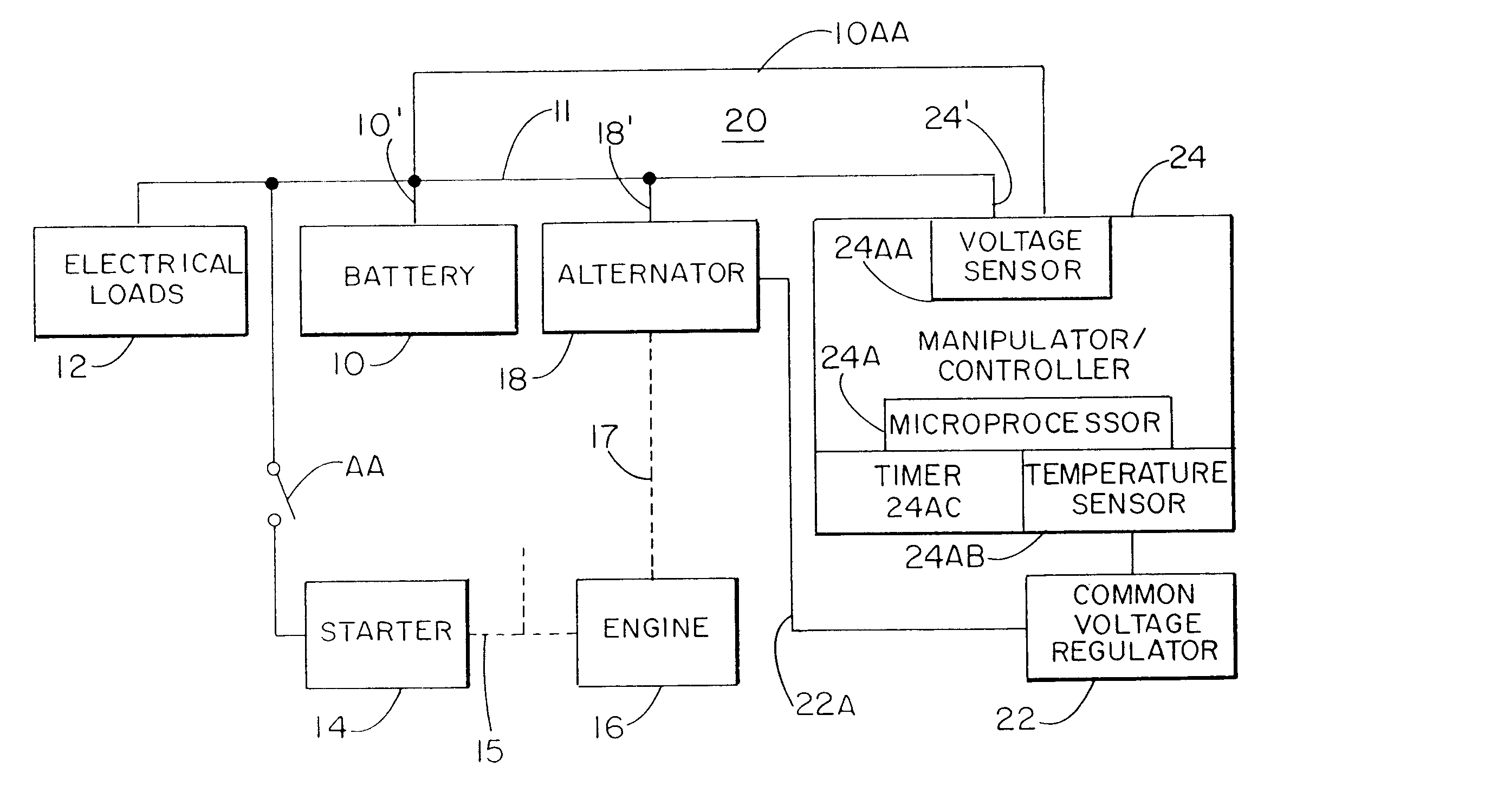
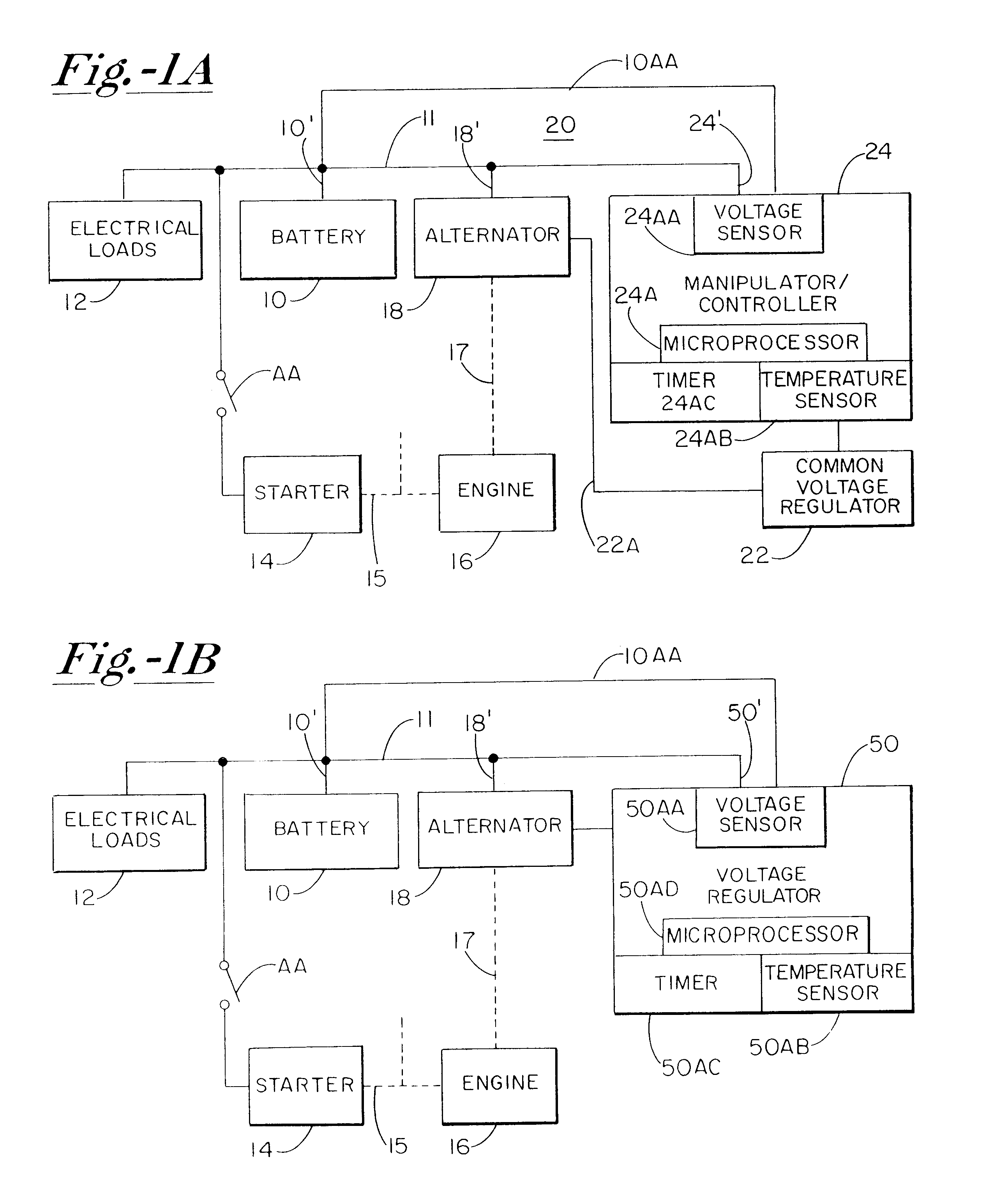
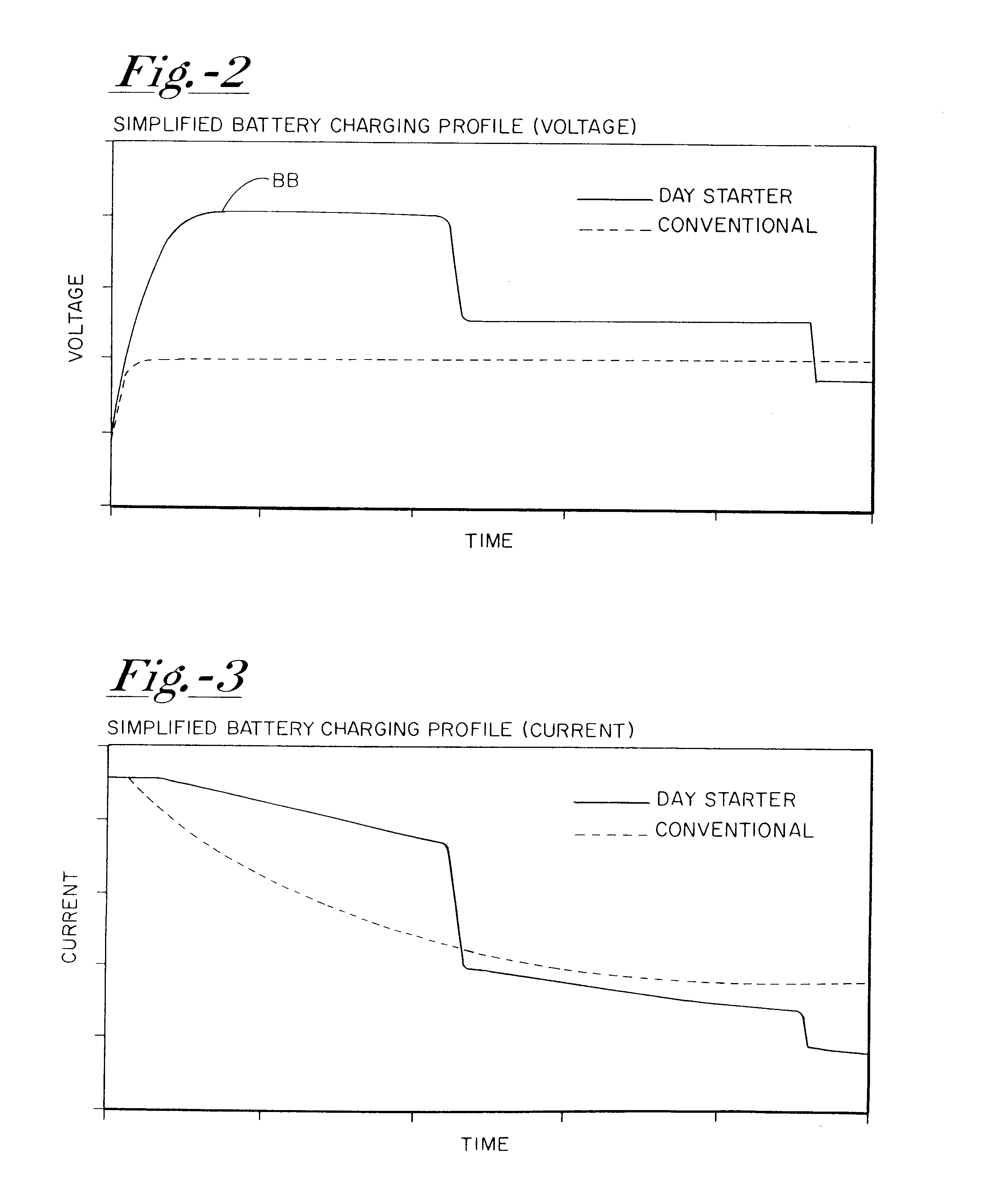
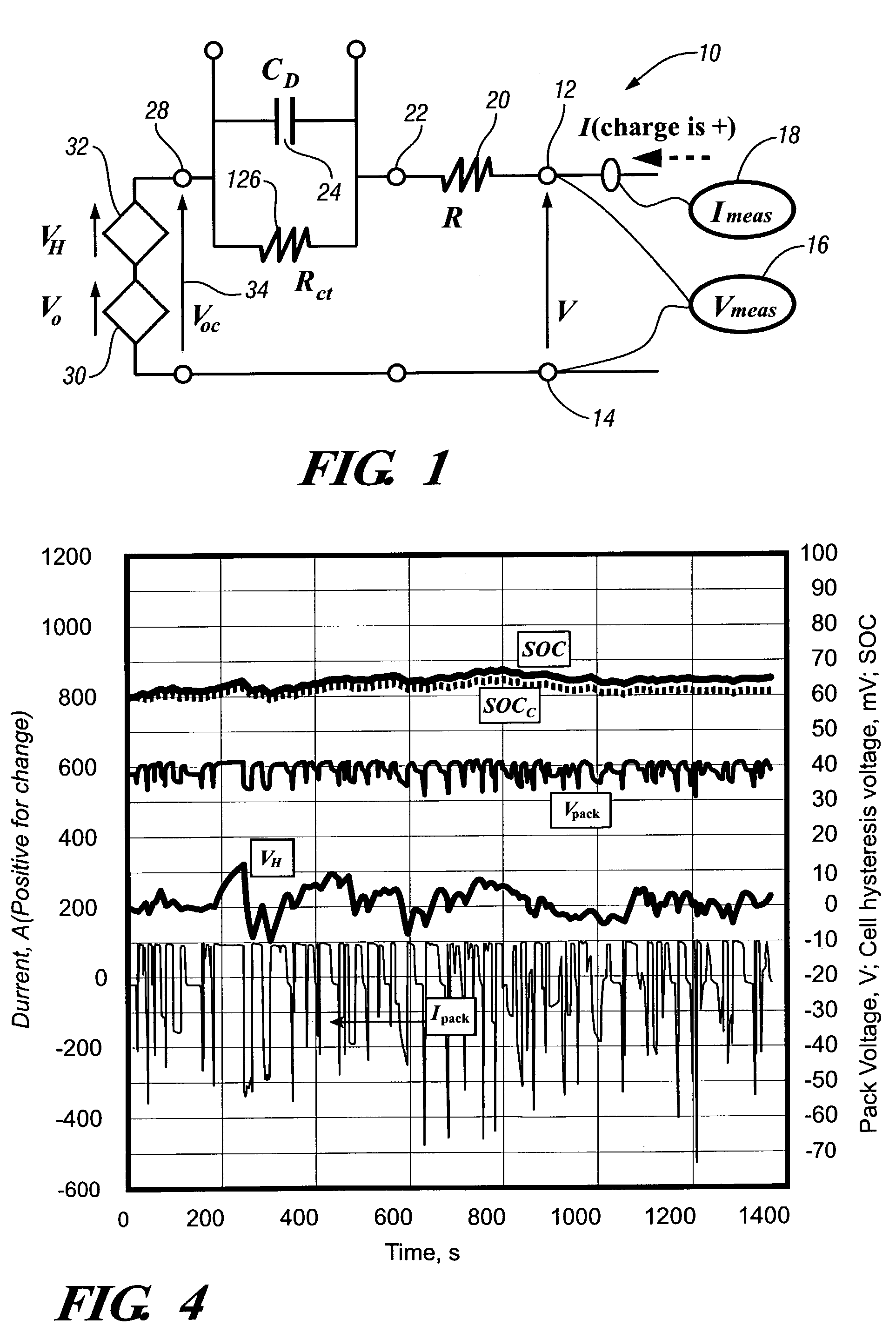
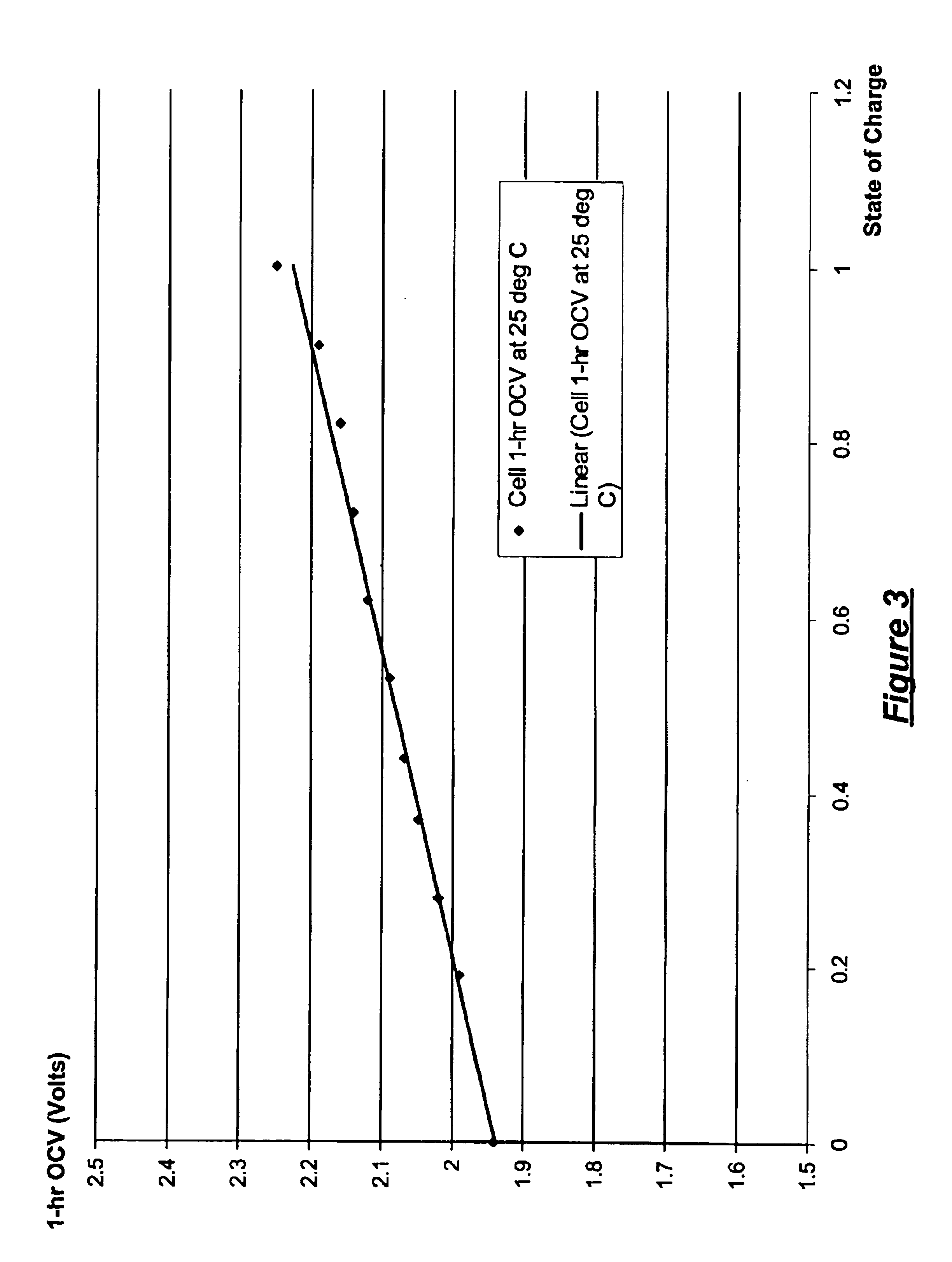
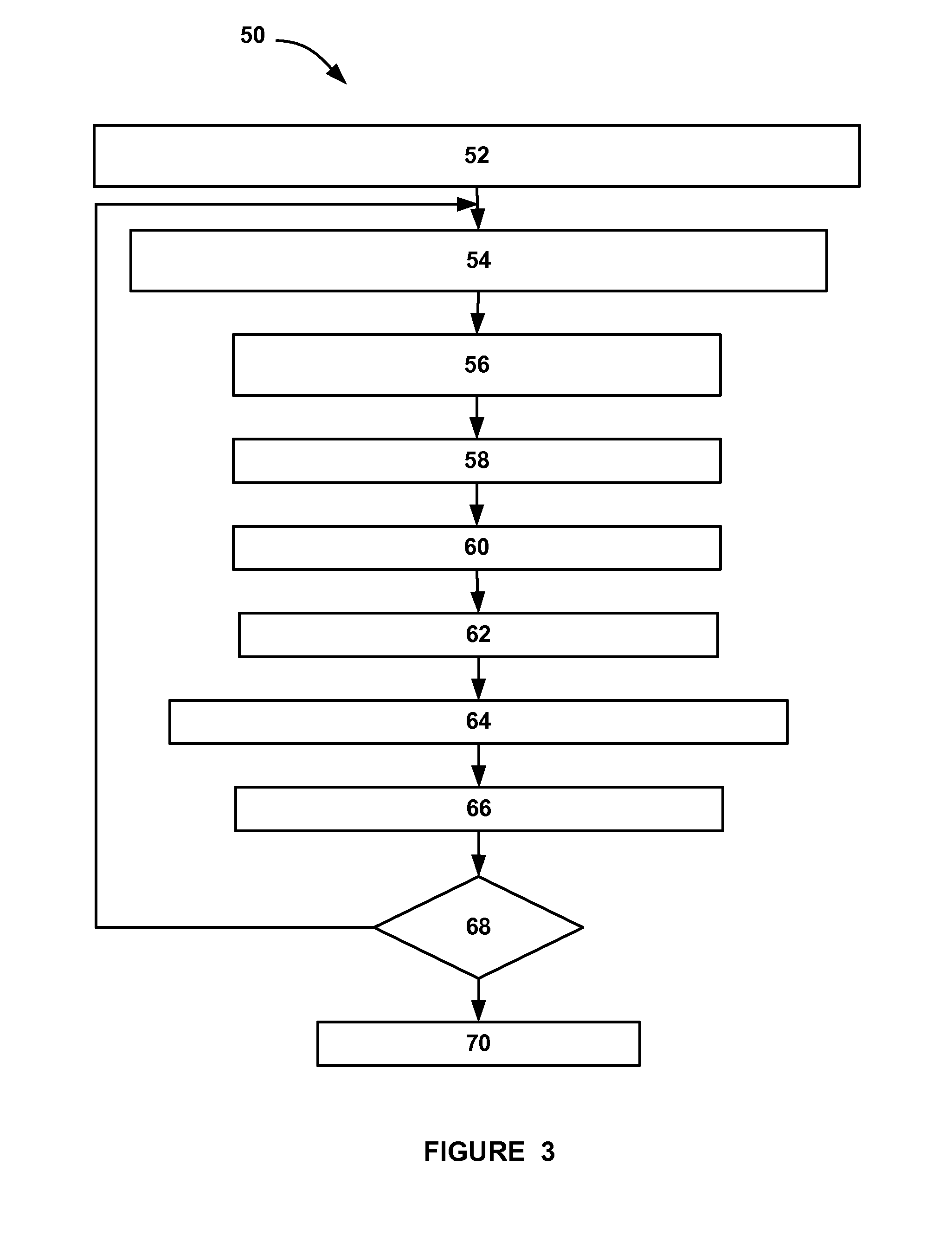
A system for actively controlling the charging profile of a battery uses a software-based alternator control unit to control the charging voltage. The system optimizes battery and alternator life. The system, on a dynamic basis, uses battery open-circuit voltage (OCV) to estimate battery state-of-charge (SOC). Also, the charging current of the battery is periodically estimated; this, after processing, provides an estimated SOC of the battery.
Owner:MIXON
Electromechanical Manipulating Device for Medical Needle and Syringe with Sensory Biofeedback and Pain Suppression Capability
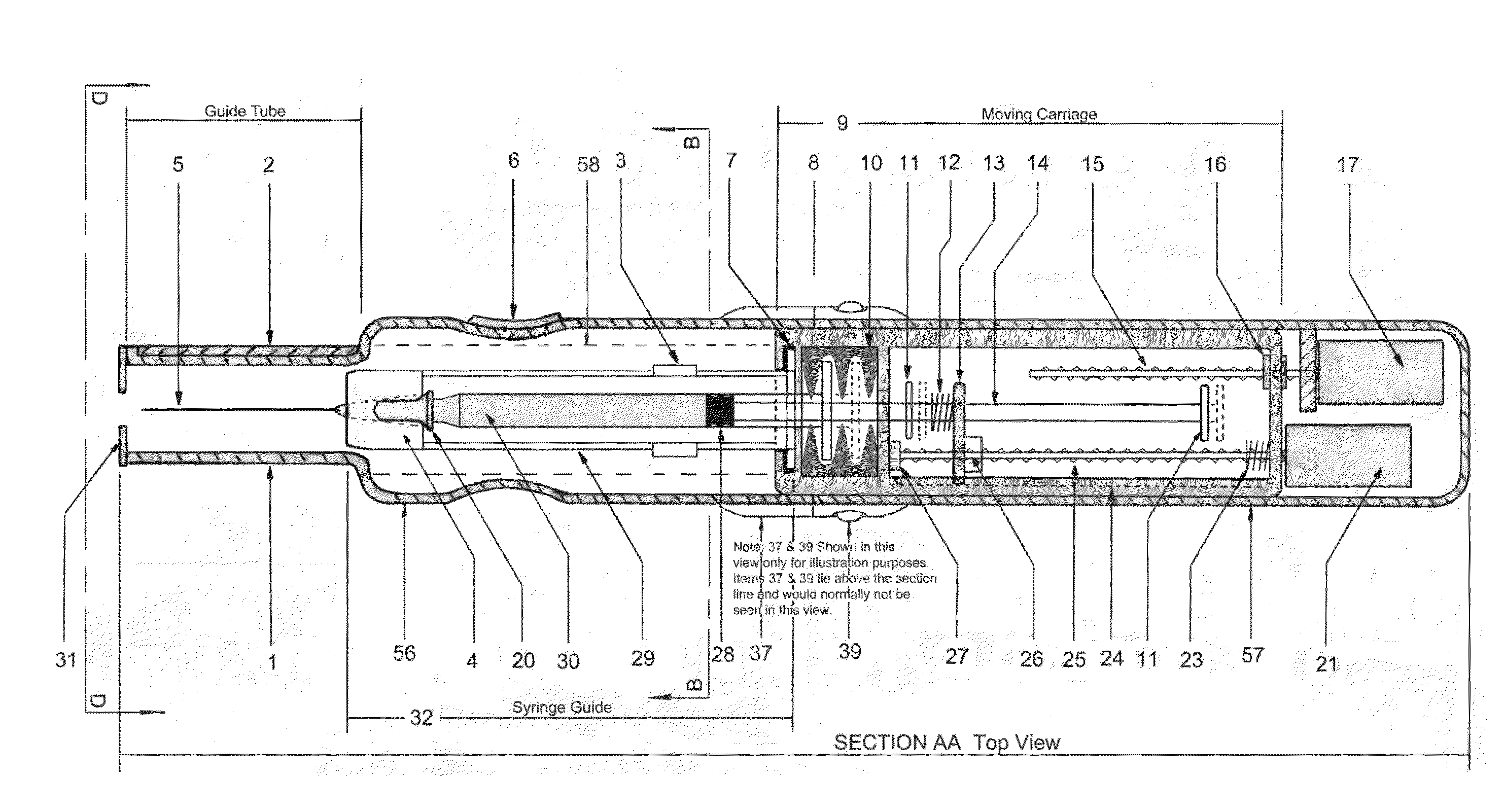
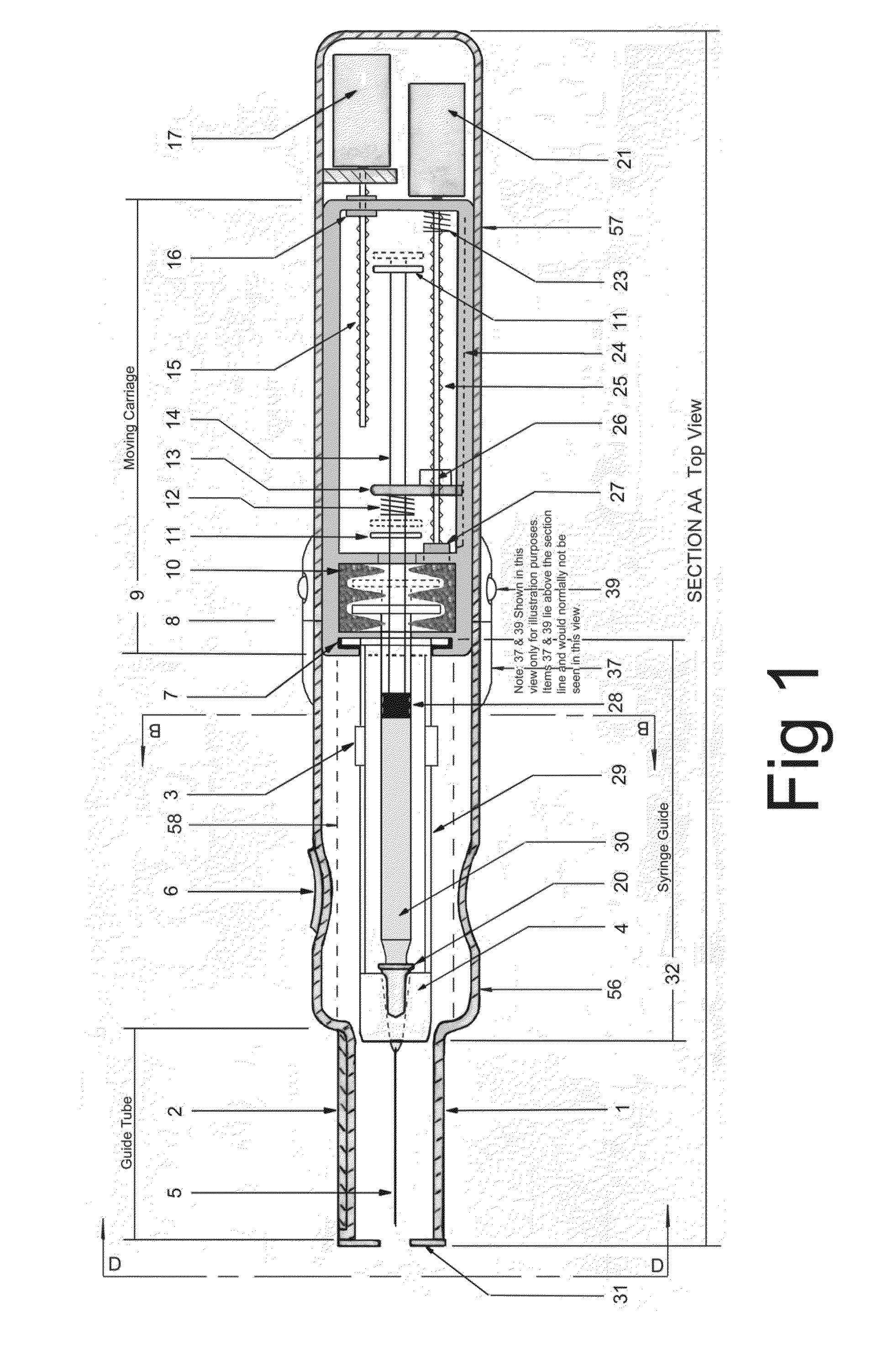
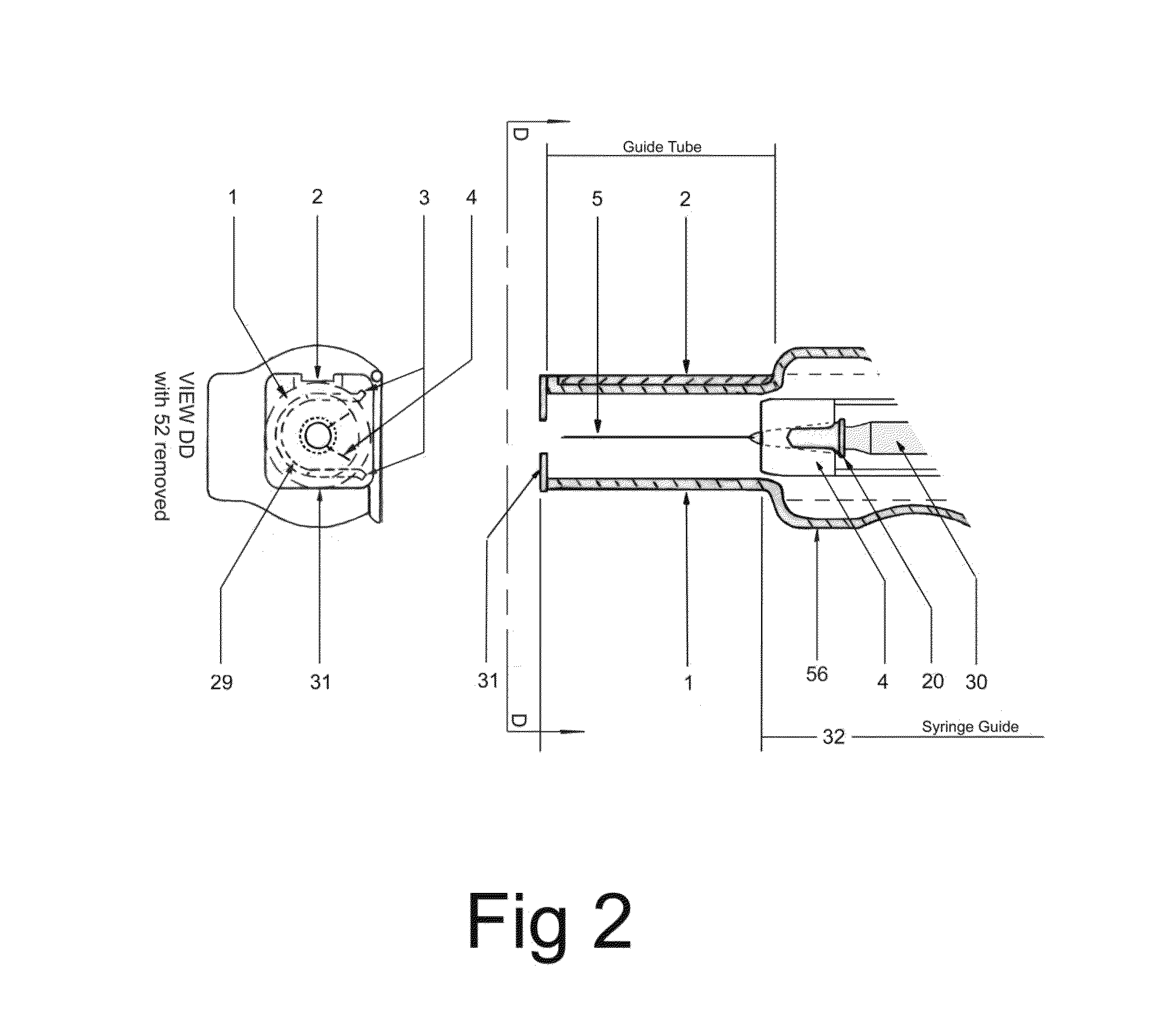
ActiveUS20140142507A1Facilitate actionDistract patientAutomatic syringesInfusion needlesBattery state of chargeDistraction
In an aspect, a medication delivering injector which includes a housing having opposing proximal and distal ends and an accessible internal cavity for inserting and removing a medicament container such as a syringe. The injector is designed to hold and manipulate a medication delivery device such as a large variety of standard and non-standard medical syringes with fixed or attached needles, assembled such that centrally located is a cylinder containing a liquid medicament and attached either permanently or removably to the distal end of the cylinder, is a hypodermic needle or cannula in fluidic communication with the cylinder. The needle can be from one-half inches to one-and-one-half inches in length in the illustrated embodiment and other sizes are possible by changes in scale of the injector. At the proximal end of the cylinder is an opening with an inserted seal or bung with an attached rod thus constituting a plunger. The central medicament containing cylinder could also be a standard medicament cartridge. Hereinafter, both are referred to as a “syringe”. A lid or access cover which, in an open position permits insertion and removal of the syringe into / from the housing in a horizontal fashion thus providing ease of loading. The syringe is inserted with the needle end toward the distal end of the housing and the plunger toward the proximal end of the housing. A movable carriage is disposed in the proximal end of the housing and slides in the axial direction forwards and rearwards such that a syringe, whose proximal end is gripped by the carriage, so moves with the carriage such that the needle exits the housing at the distal end of the housing and pierces the tissue of the patient prior to dispensing of the medicament, and then is retracted after the medicament has been dispensed by retraction of the carriage. Attached to the carriage is an actuator which pushes on the syringe plunger causing the medicament contained within the syringe cylinder to be dispensed through the needle into the patient's tissue. In one embodiment suitable for both removable needle and fixed needle syringes, the proximal end of the syringe including the syringe finger flange is gripped by an elastomeric flange grip which resides within the carriage, while the syringe distal end resides in and is supported by a “syringe guide” which is attached to the carriage and thus moved with it. In another embodiment which is so made to accommodate syringes with removable needles and their safe disposal, the syringe flange at the proximal end of the cylinder is gripped by an elastomeric flange grip which resides in the carriage as described above, while the syringe distal end is supported by a needle which resides in a removable disposable needle shield. The needle guide is biased toward the carriage and thus, the syringe body is compressed and guided as the carriage moves forward and rearward.Both the movement of the carriage and the actuator are controlled by servo motors which are controlled by electronics and a microcontroller so operating such that speeds and accelerations are controlled smoothly and gently so as to avoid the stop / start motion of motors controlled by limit switches and simple electronics or the vibration and abruptness such as result from injectors powered by compressed springs or gas. Furthermore, the forces are adaptive to the loads imposed and the requirements necessary for the proper dispensation of medicaments with high viscosity or sensitivity to shear forces.The housing, approximately midway between proximal and distal ends, is affixed by a hinge such that the device can be folded in half to provide for a more compact device to be stored and transported.On the distal end of the housing are electrical sensor pads in communication with the microcontroller such that contact with the patient's skin and the angle at which the injector is held against the skin and the steadiness with which the injector is being held can be ascertained. Within the housing are a haptic vibrator and an audio speaker, both producing a vibration which is variable in pitch in such a manner that biofeedback is provided to the injector user as to the pressure, angle and steadiness with which they are holding the injector against the skin. This facilitates the action of injection oneself in the gluteus muscle where visual feedback isn't available to the patient.Multiplexed onto the electrical sensor pads, is a TENS (Transcutaneous Electrical Nerve Stimulation) generator which is operational (at the user's choice) just before and during the injection to interrupt or quench the pain of tissue perforation often accompanied with needle injections.Contained on the surface of the housing such as on the access cover, is a display such that user menus, device state, directions, and battery charge status, etc. are displayed. Also contained on the surface of the housing, on the proximal half, are buttons which control the menus and selections that are shown on the display. These selections provide for the user to set such parameters as hypodermic insertion speed and medicament dispensing speeds, the preferred mode of user biofeedback which can include: speech mode (which can be accompanied by musical themes and ringtones, plus variable pitch tone and haptic vibration), MP3 mode (which has speech muted but includes musical ringtones and variable pitch tone and haptic vibration), and haptic mode (which is haptic vibration and audio tone queues needed for injector position biofeedback and readiness), plus mute mode (which provides no audio but the haptic vibration remains), and none, which provides no vibrational biofeedback, yet the display information always remains available.Also contained on the surface of the housing on the distal half, is an “injection initiate” button which causes the sequences required for performance of an injection to occur if said button is “enabled”.Contained within the electronics and its operating program is the ability to audibly play pre-recorded human speech in any language such that: directions in the form of consecutive steps which are required to load the medicament container (syringe) into the device, consecutive steps to perform an injection, steps to remove and properly dispose of parts, to alert of device status and menu choices, etc. can be played through the audio speaker. Also contained within the electronics and program is the ability to play musical ringtones as a distraction during the needle insertion and injection or when a scheduled injection alarm is reached, or calming human voice exhibiting bedside manner during the needle insertion and injection.Also contained within the electronics and software is a real-time clock-calendar which can store a patient's injection schedule and play a musical ringtone as an alarm as each scheduled injection becomes due. Also contained within the electronics and software are the ability to communicate with a personal computer through a USB port, which can also charge the injector's rechargeable battery. The battery in one embodiment, consists of three AAA batteries which can be rechargeable or non-rechargeable. The USB port in combination with an application running on the personal computer, is used to download the injection schedule into the real-time clock-calendar and to download ringtones and musical themes of the user's choice and to download foreign language sets for the pre-recorded human language feature.The injector can be further equipped with the capability to aspirate the tissue by drawing back on the plunger thus creating a vacuum into which fluids will flow. These fluids enter the syringe cylinder where they can be checked for the presence of blood by optical absorption in the red spectrum. This information is useful in the instances where intramuscular injections are to be given with drugs whose ‘Full Prescribing Information’ instructs the patient to aspirate and check for blood in the syringe which indicates that the puncture of a vein has occurred, and if so detected, to abort the injection and then re-inject into a different location.
Owner:INTELLIPEN INC
Method and apparatus for generalized recursive least-squares process for battery state of charge and state of health
InactiveUS7324902B2Current/voltage measurementTesting electric installations on transportBattery state of chargeState of health
Owner:GM GLOBAL TECH OPERATIONS LLC
Simple optimal estimator for PbA state of charge
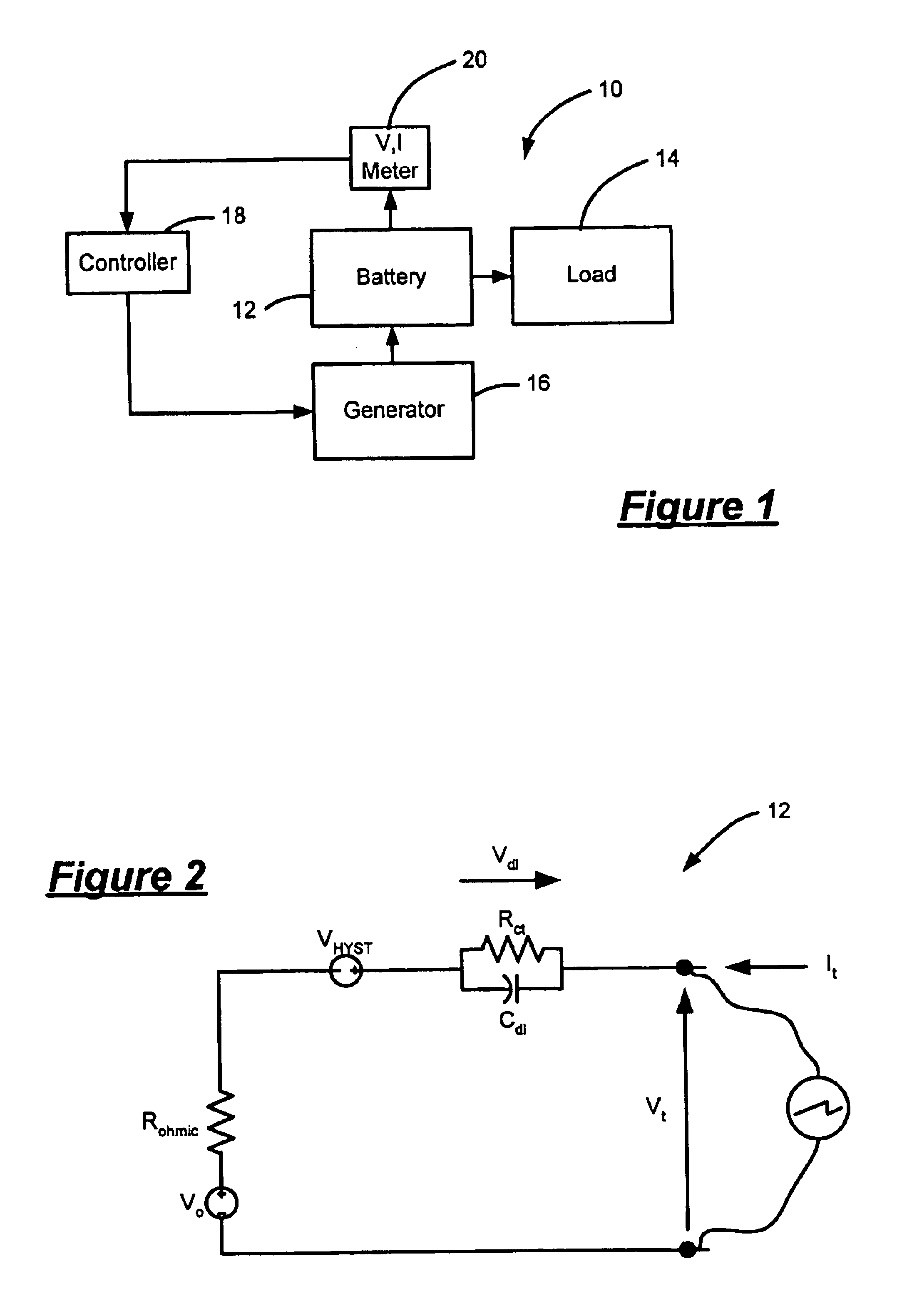
InactiveUS6927554B2Batteries circuit arrangementsCurrent/voltage measurementBattery state of chargeTerminal voltage
A state of charge estimator for a battery includes a meter that generates a terminal voltage signal of the battery and a terminal current signal of the battery. A controller employs a linearized model of the battery and a time-varying state estimator. The controller process a synthesized input based on the terminal current and the terminal voltage to estimate the battery state of charge.
Owner:GM GLOBAL TECH OPERATIONS LLC
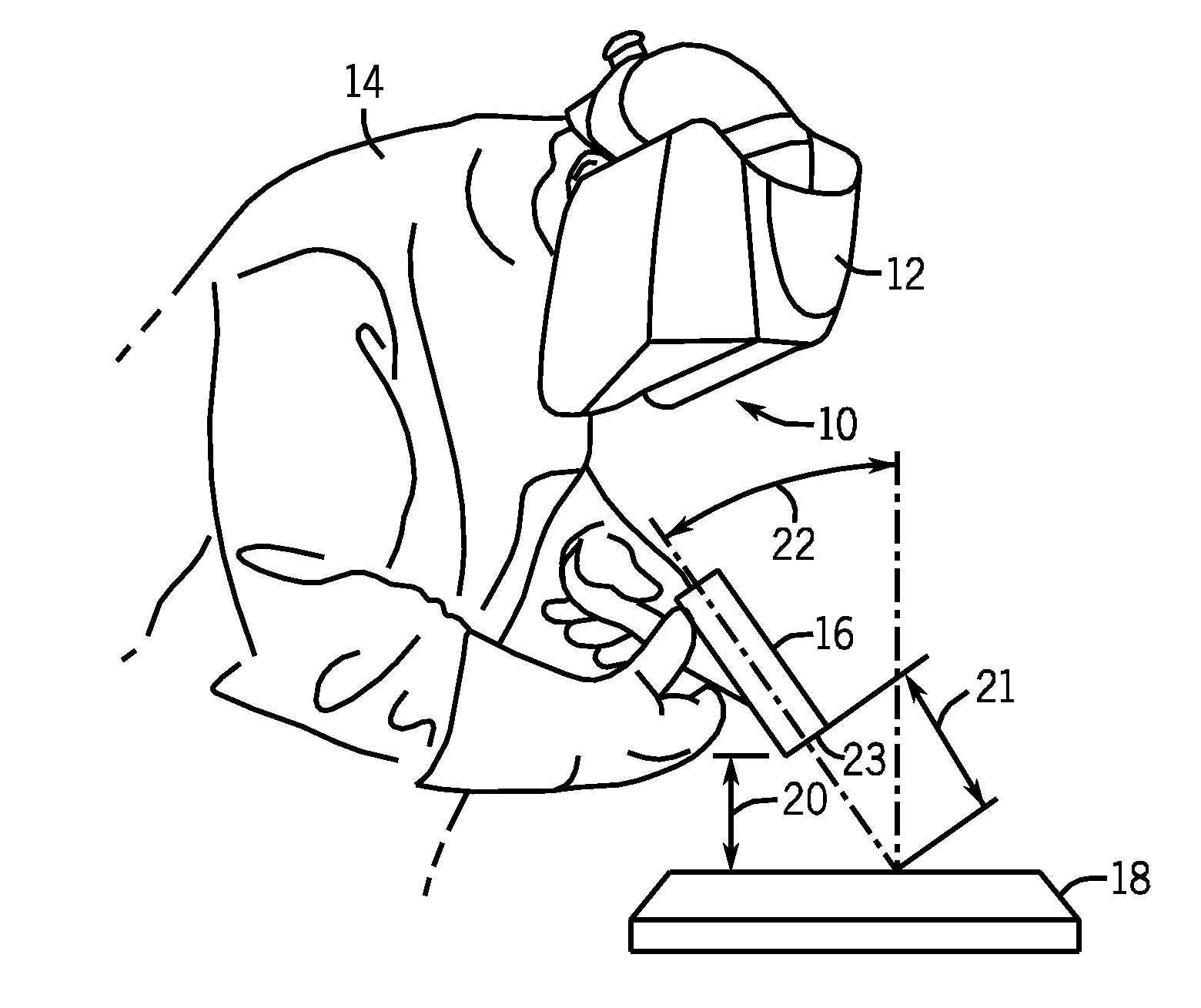
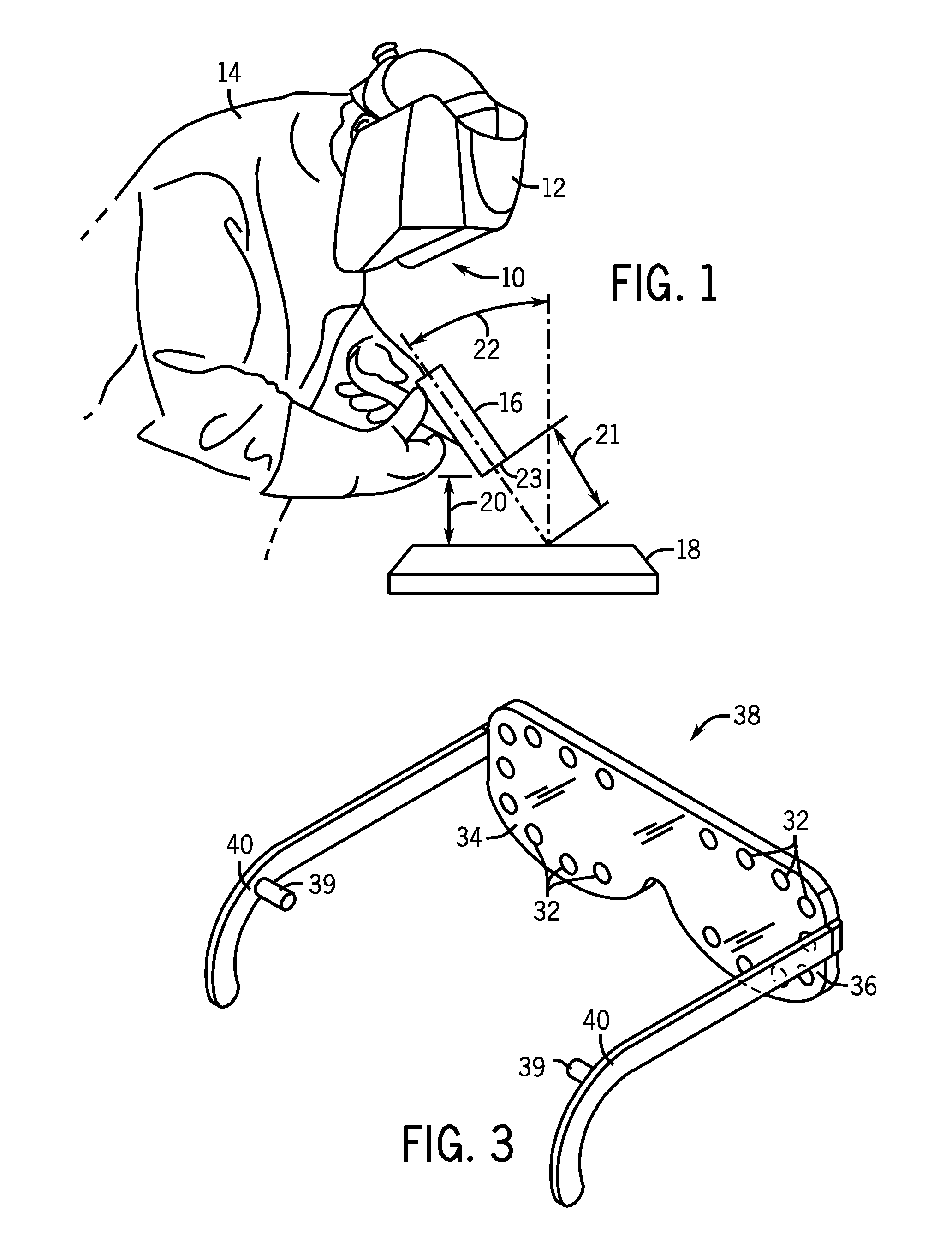
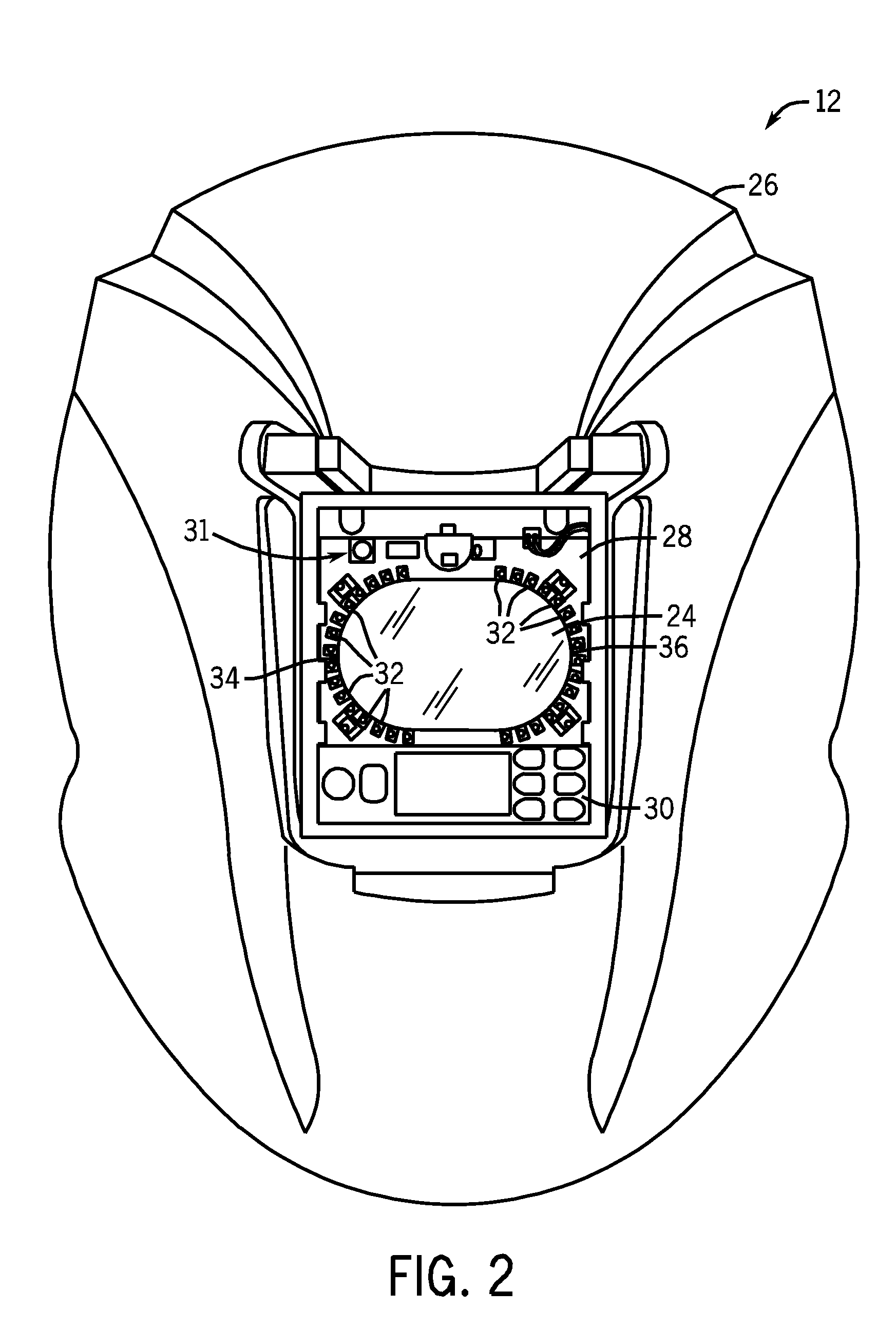
Weld characteristic communication system for a welding mask
Methods and systems for transmitting a torch angle and / or a torch-to-workpiece distance error to a welding operator when these parameters are outside of a preset optimal range via real time visual and / or audio cues are provided. One embodiment of the present disclosure relates to weld characteristic communication via intuitive arrays of visual indicators located on the periphery of a lens, which indicate to the welding operator the direction and severity of the torch angle error. In one embodiment, audio cues, such as pulsed or continuous tones may be used to communicate torch-to-workpiece distance to the welding operator. In certain embodiments, vertical visual indicator arrays may be used to indicate additional weld or auxiliary information, such as battery charge state, torch speed and so forth, to the welding operator. All the components of the communication system may be located in or on the welding helmet or the components may be split between the helmet and a belt pack.
Owner:ILLINOIS TOOL WORKS INC
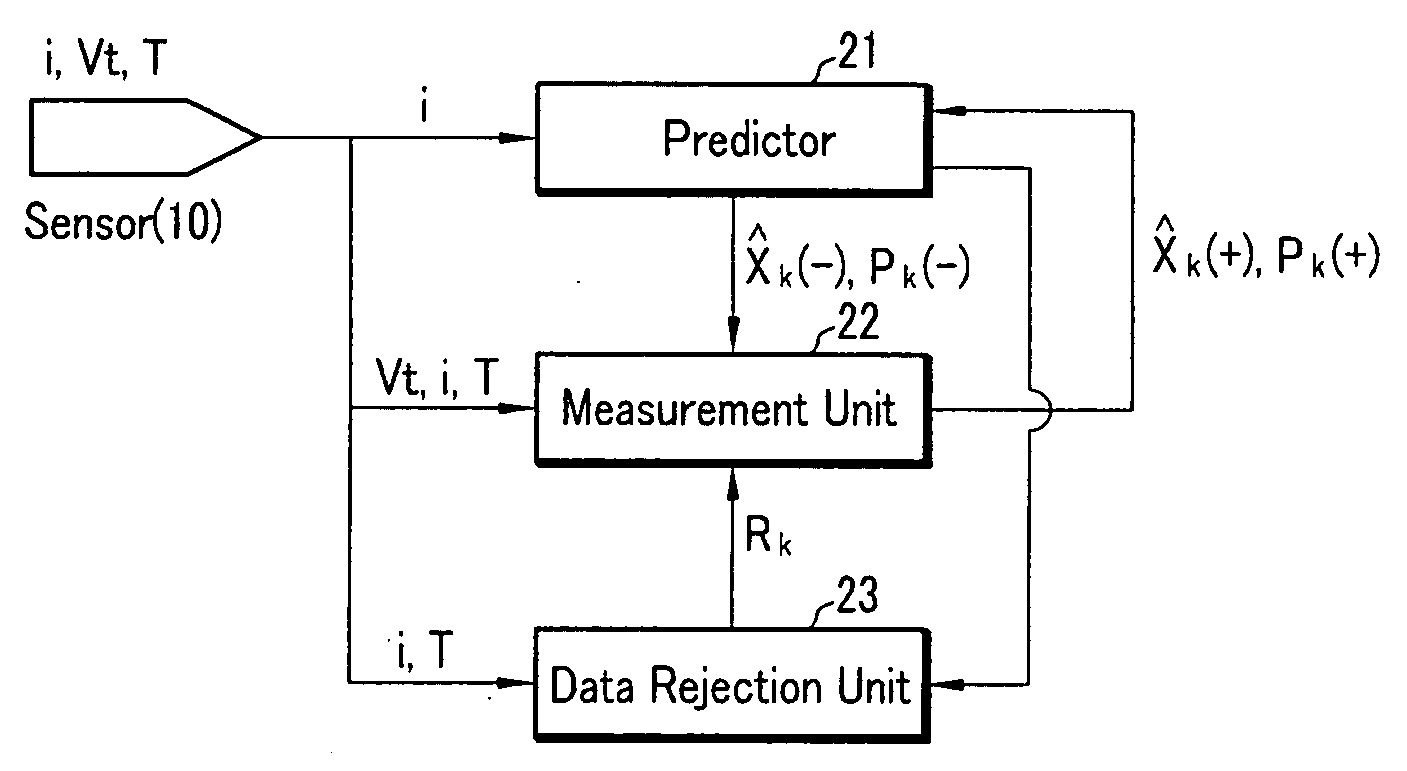
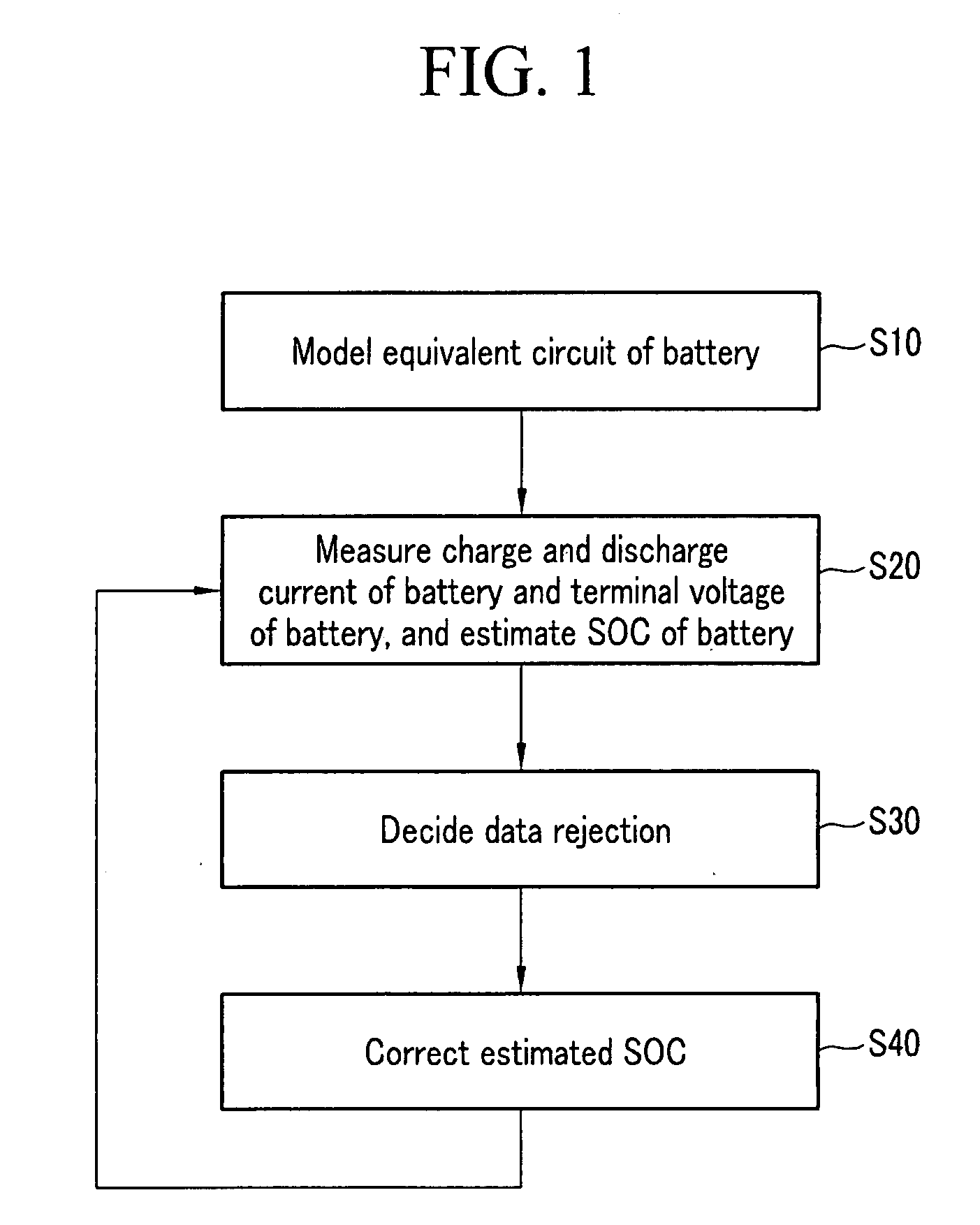
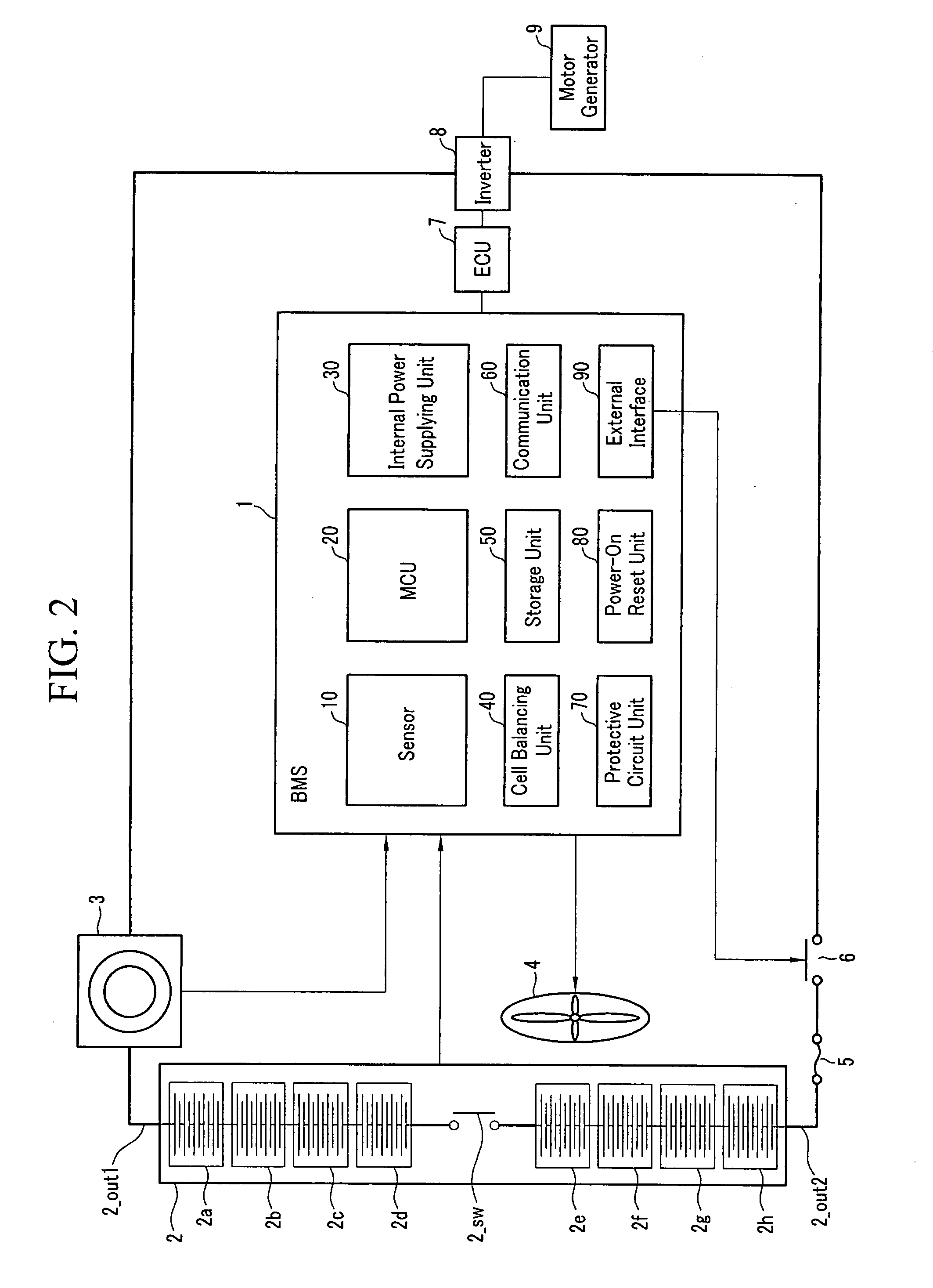
Method for estimating state of charge of battery, battery management system using same, and driving method thereof
ActiveUS20070299620A1Reduce calculationAccurately estimate SOCBatteries circuit arrangementsOperating modesBattery state of chargeElectrical battery
A battery management system using a measurement model modeling a battery, and estimating a SOC (state-of-charge) of the battery, and a battery driving method thereof. The battery management system is constructed with a sensor, a predictor, a data rejection unit, and a measurement unit. The sensor senses a charging and discharging current flowing through the battery, a temperature of the battery, a terminal voltage of the battery. The predictor counts the charging and discharging current, and estimates the state-of-charge of the battery. The data rejection unit generates information associated with an error generated from the measurement model, as a function of at least one of the battery temperature, the charging and discharging current, the state-of-charge, and a dynamic of the charging and discharging current. The measurement unit corrects the estimated state-of-charge of the battery, using the measurement model and the information associated with the error.
Owner:SAMSUNG SDI CO LTD +1
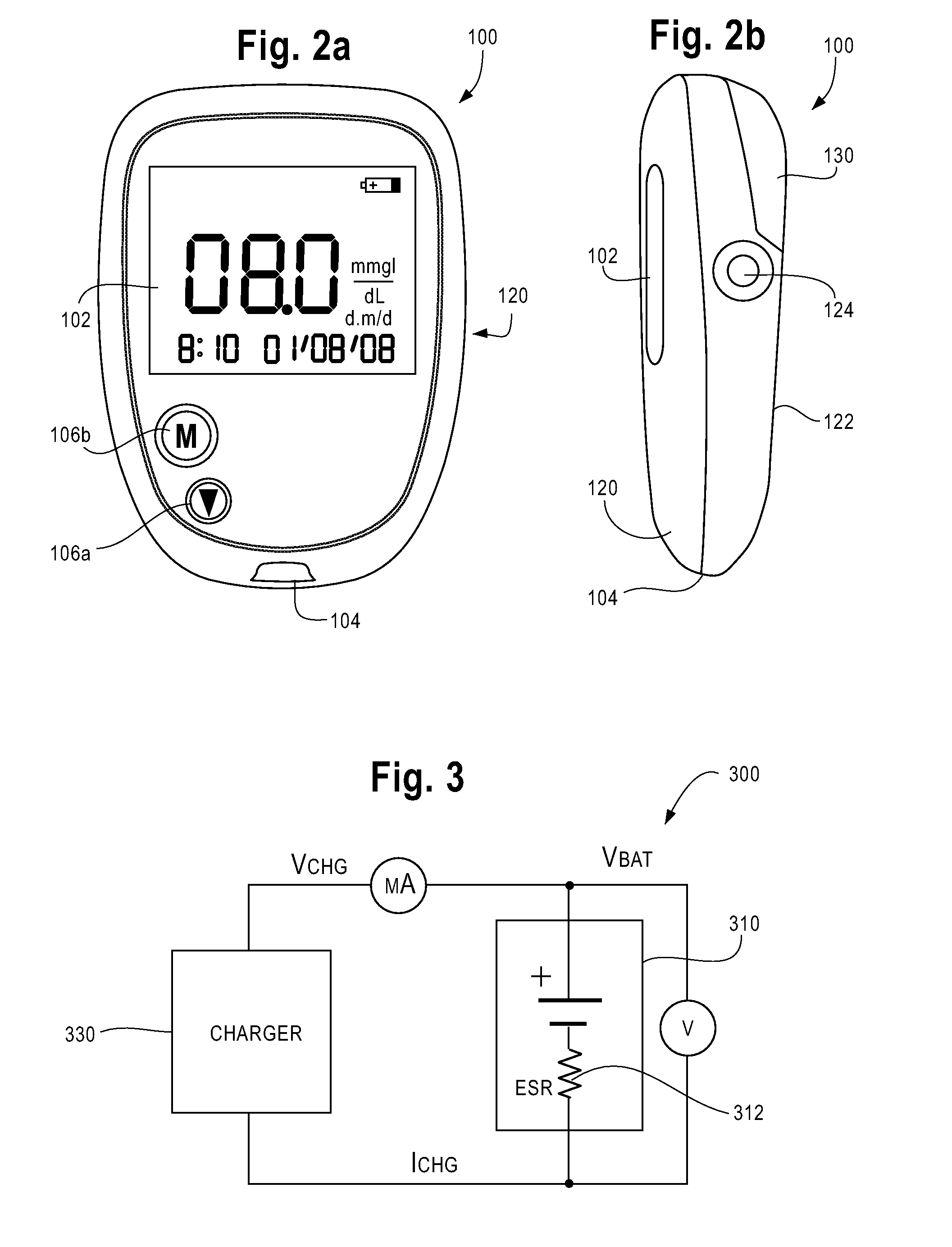
Rapid Charging And Power Management Of A Battery-Powered Fluid Analyte Meter
ActiveUS20090146826A1Rapid charge algorithmFast chargingElectrical testingSecondary cells charging/dischargingBattery state of chargeElectrical battery
A system and method is described for rapid charging and power management of a battery for a meter. A charger component is operably associated with the meter and is capable of executing a rapid charge algorithm for a rechargeable battery. The algorithm includes monitoring for a connection to an external power source and implementing a charging routine of a battery at a first charge rate and then at a second charge rate. The second charge rate is lower than the first charge rate. A temperature rise in the rechargeable battery due to the first charge rate has a negligible heat transfer effect on the fluid sample. The meter can also include a power switch for controlling current flow to a battery fuel gauge. The power switch is open when the meter enters into a sleep mode. The state of battery charge is determined after the meter exits the sleep mode.
Owner:ASCENSIA DIABETES CARE HLDG AG
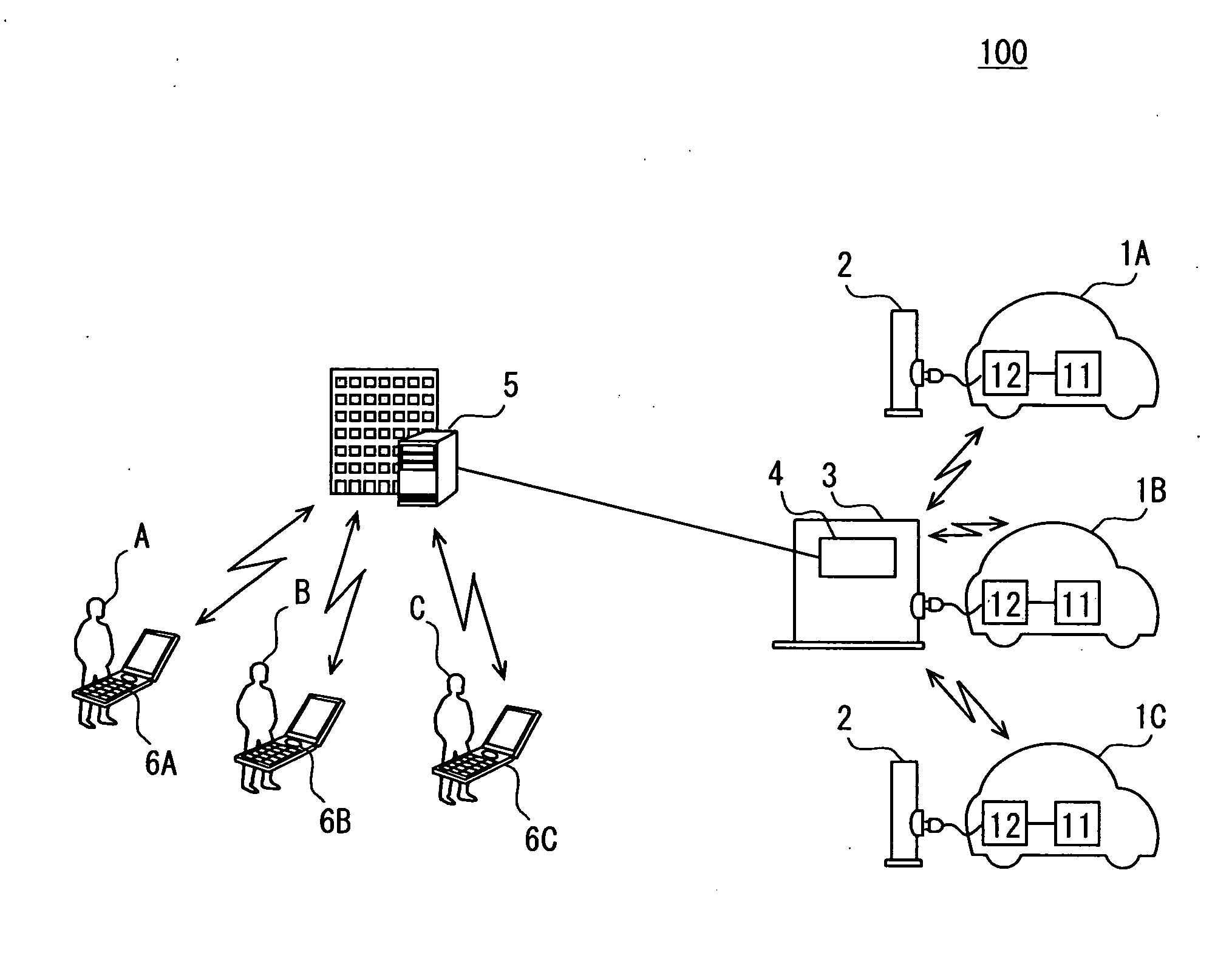
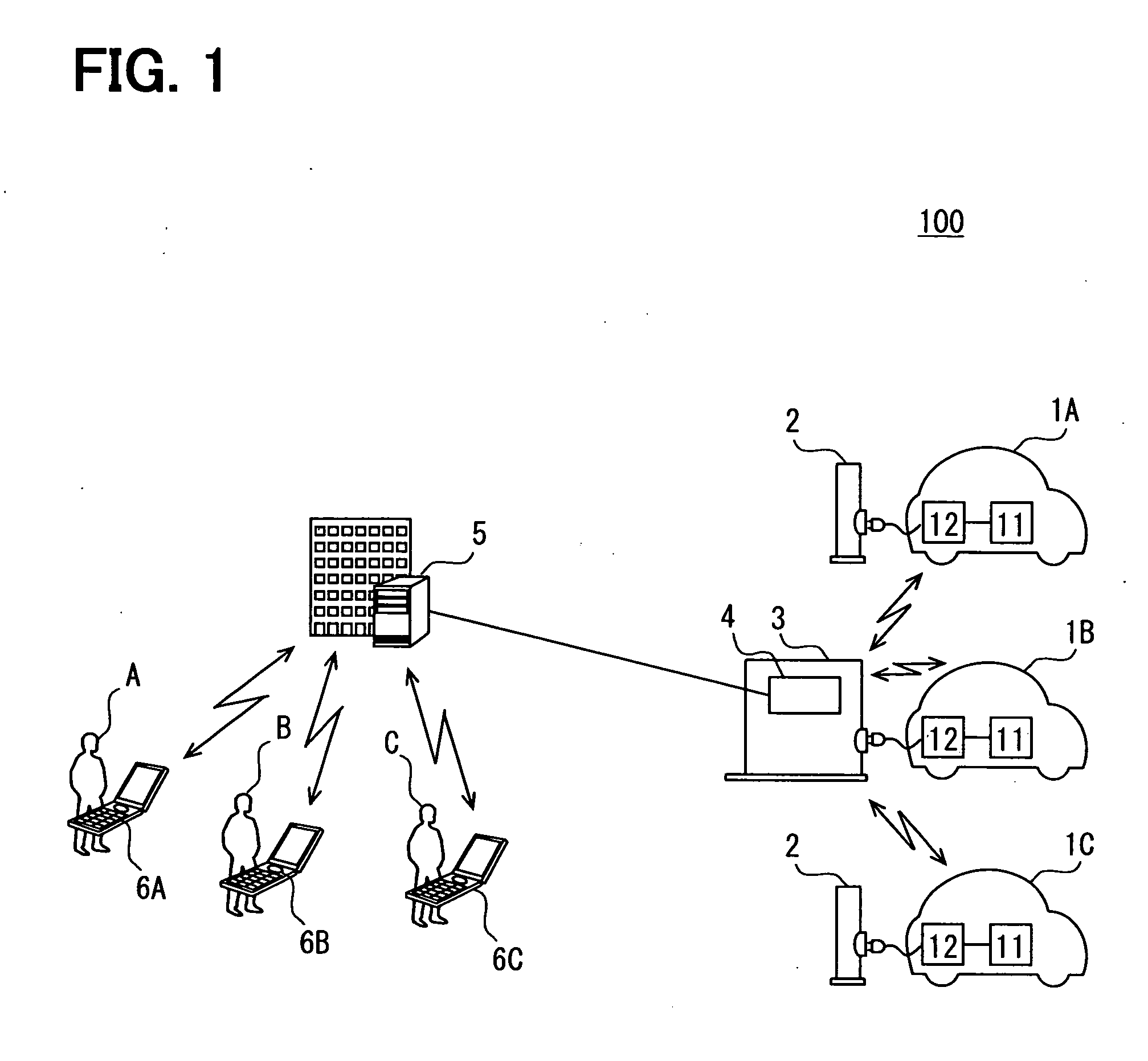
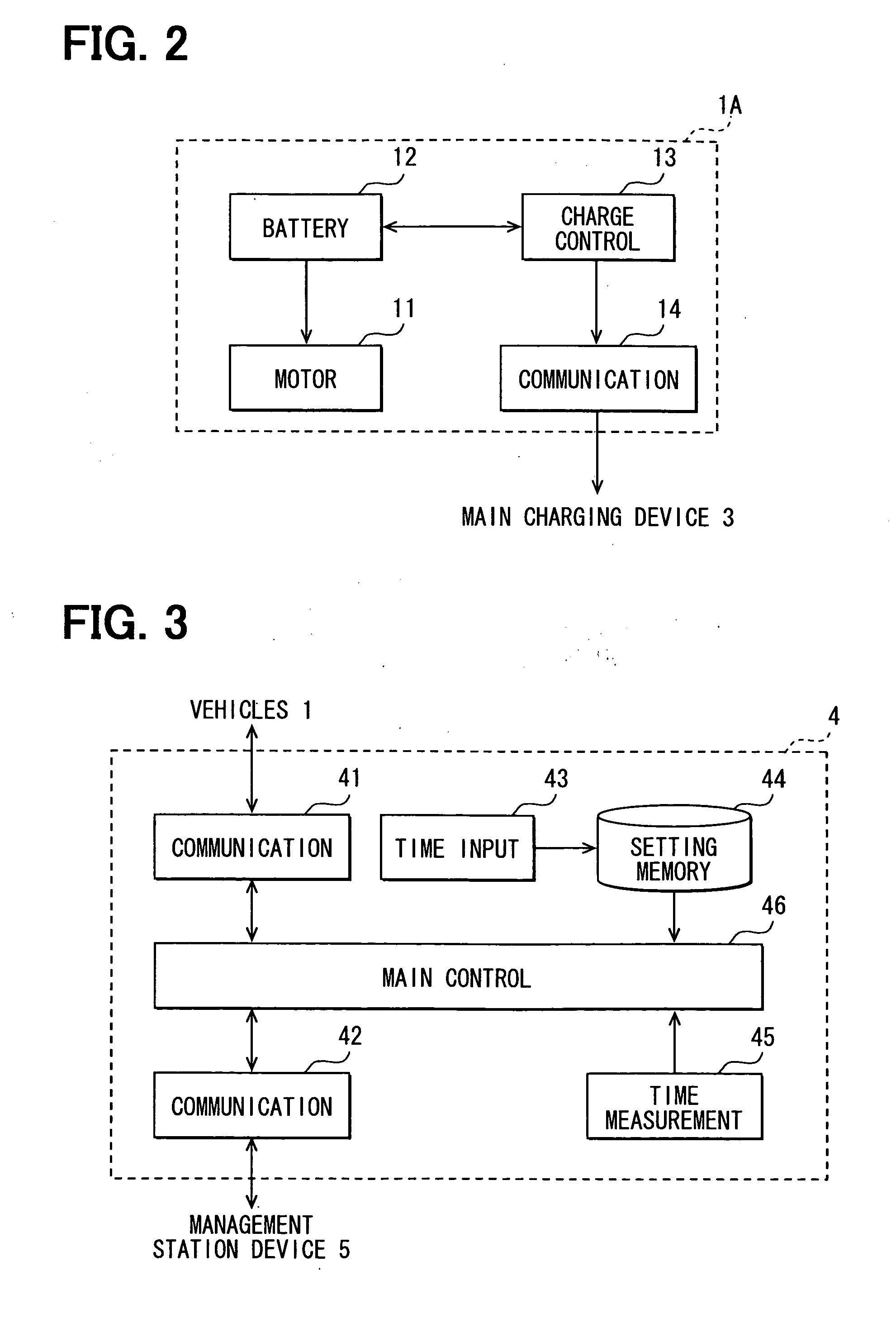
Battery charge state transmission device and external charging system
InactiveUS20110057612A1Reduce the amount of communicationReduce the amount requiredBatteries circuit arrangementsCharging stationsBattery state of chargeBattery charge
A communication part of a battery charge state transmission device of a charging device acquires charge state information of each external charge vehicle. A station communication part transmits the charge state information to an information management station device. The charge state information is grouped for transmission so that the number of transmissions, the amount of communication traffic and the cost of communication with the management station device, and the operation load of the management station device are reduced.
Owner:DENSO CORP
Systems, Methods and Circuits for Determining Micro-Short
ActiveUS20090099799A1Batteries circuit arrangementsMaterial analysis by electric/magnetic meansBattery state of chargeEngineering
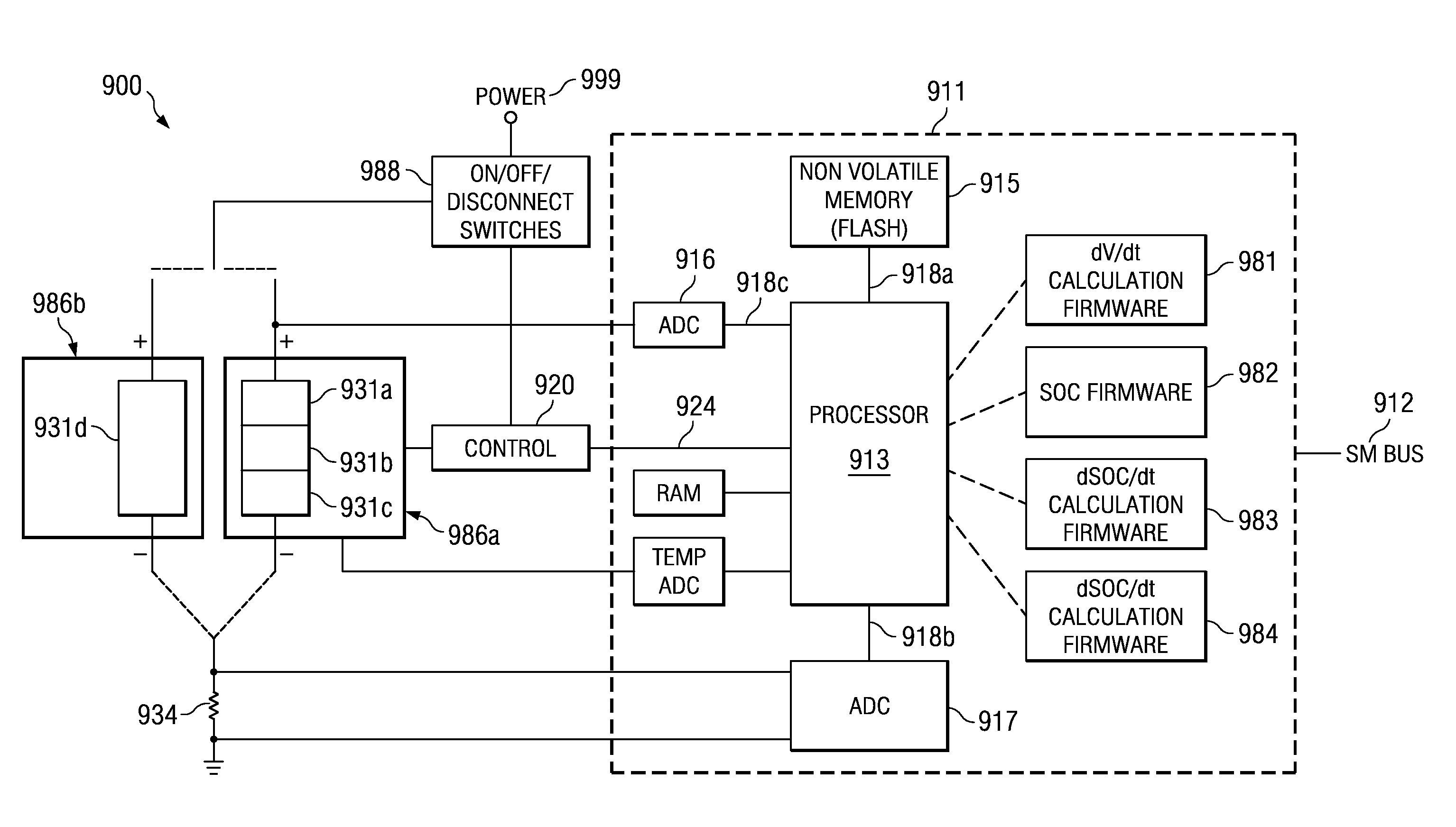
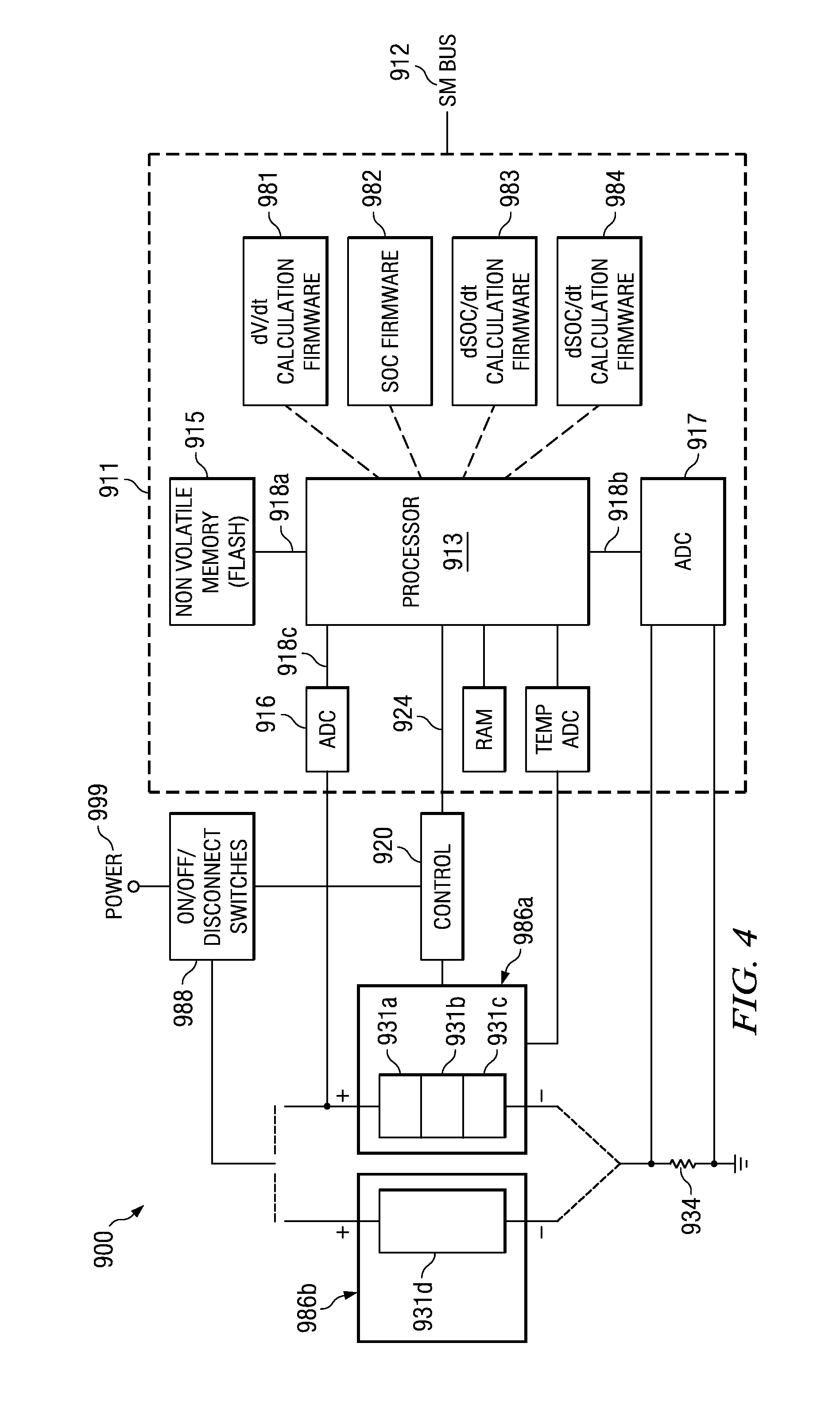
Various systems and methods for determining micro-shorts are disclosed. For example, some embodiments of the present invention provide battery systems including a determination of potential micro-shorts based on rate of change of state of charge. Such battery systems include: a battery, a processor, and a computer readable medium. The computer readable medium includes instructions executable by the processor to: determine a rate of change of the state of charge of the battery; compare the rate of change of the state of charge of the battery against a threshold; and indicate a potential failure where the result of the comparison is beyond the threshold.
Owner:TEXAS INSTR INC
Adaptive battery parameter extraction and soc estimation for lithium-ion battery
ActiveUS20110309838A1Reliable battery state-of-charge estimateMaterial analysis by electric/magnetic meansElectrical testingBattery state of chargeElectrical battery
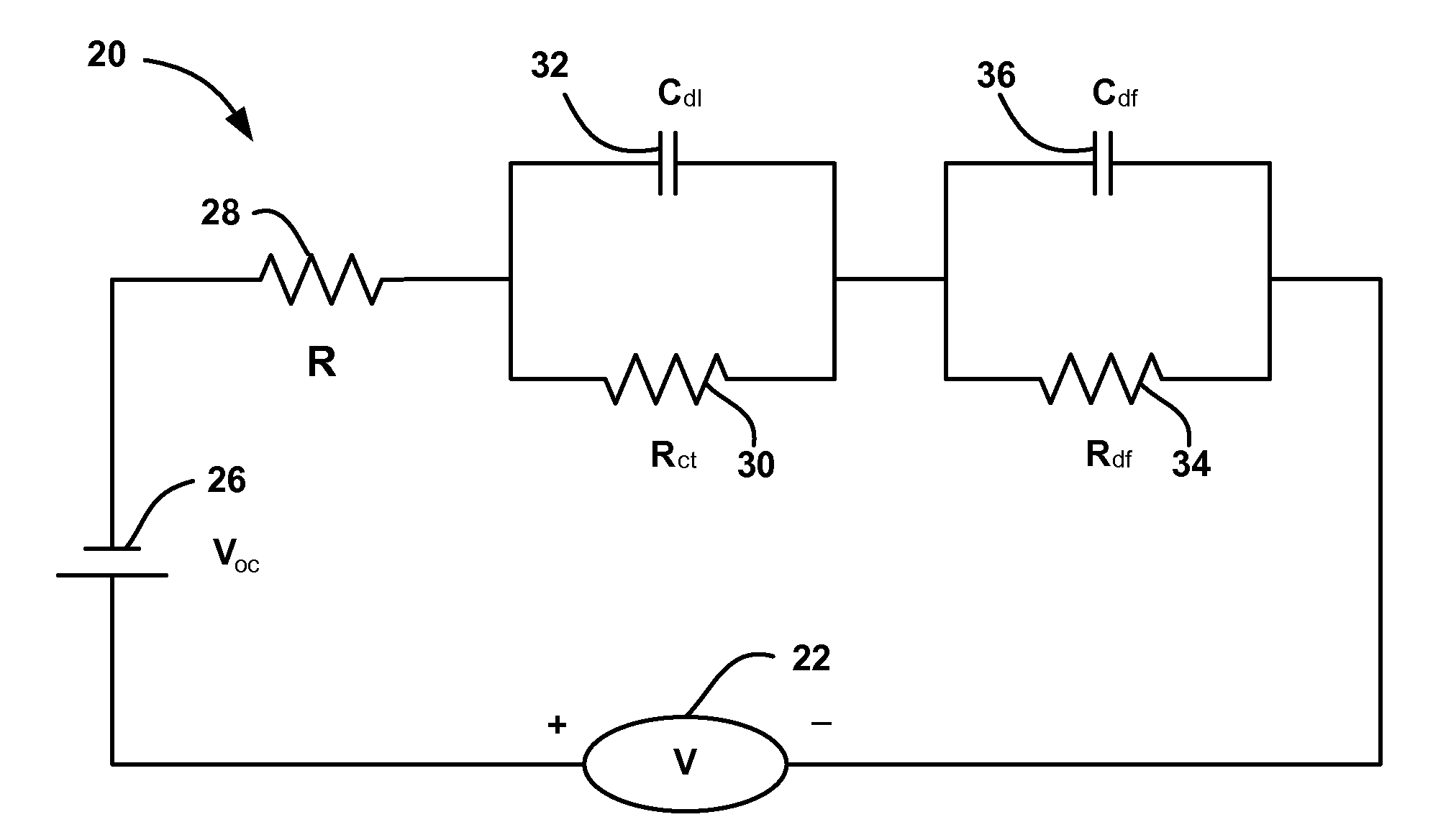
A system and method for estimating internal parameters of a lithium-ion battery to provide a reliable battery state-of-charge estimate. The method uses a two RC-pair equivalent battery circuit model to estimate the battery parameters, including a battery open circuit voltage, an ohmic resistance, a double layer capacitance, a charge transfer resistance, a diffusion resistance and a diffusion capacitance. The method further uses the equivalent circuit model to provide a difference equation from which the battery parameters are adapted, and calculates the battery parameters from the difference equation.
Owner:GM GLOBAL TECH OPERATIONS LLC
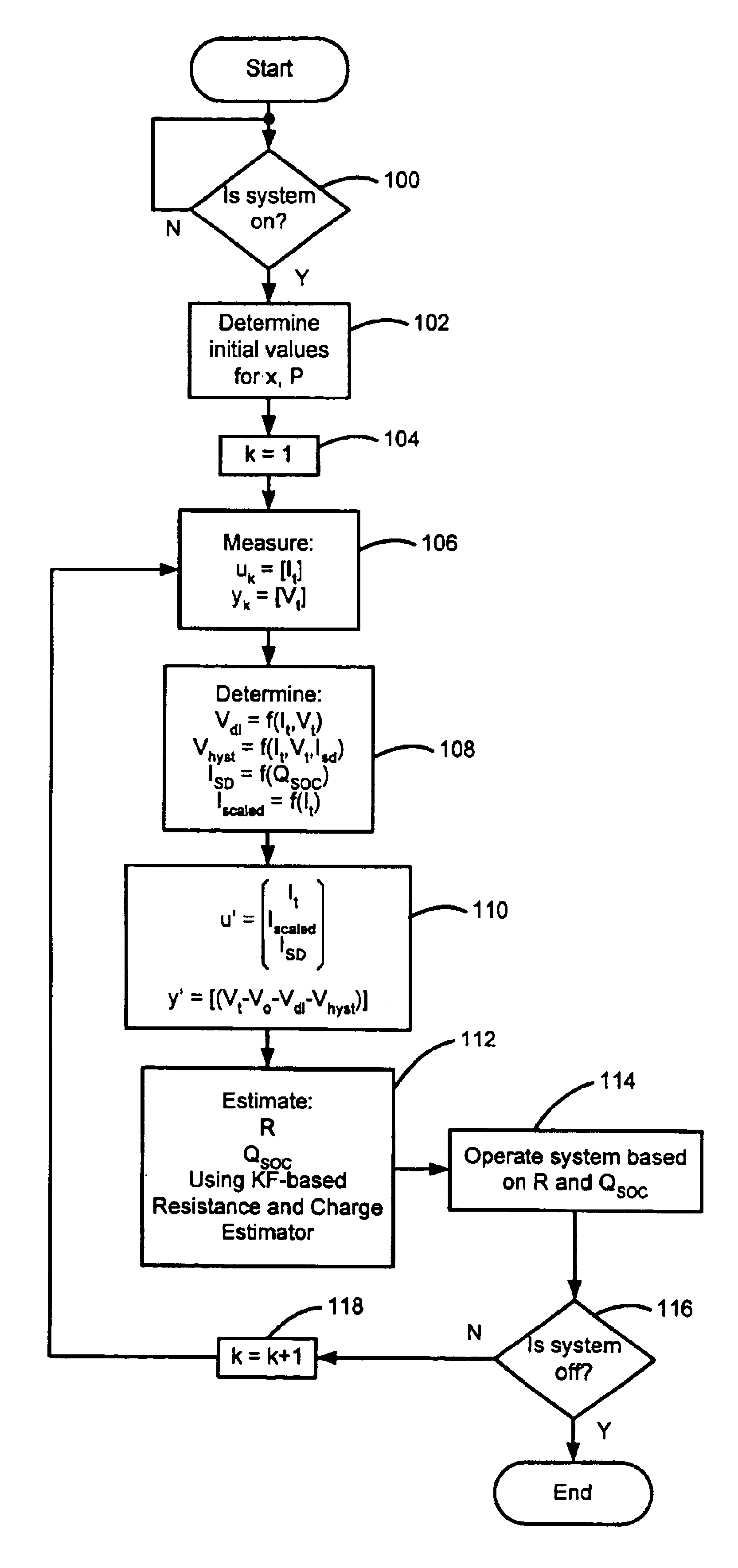
Battery state-of-charge estimator
InactiveUS20050269991A1High precisionCircuit monitoring/indicationElectric devicesBattery state of chargeElectrical resistance and conductance
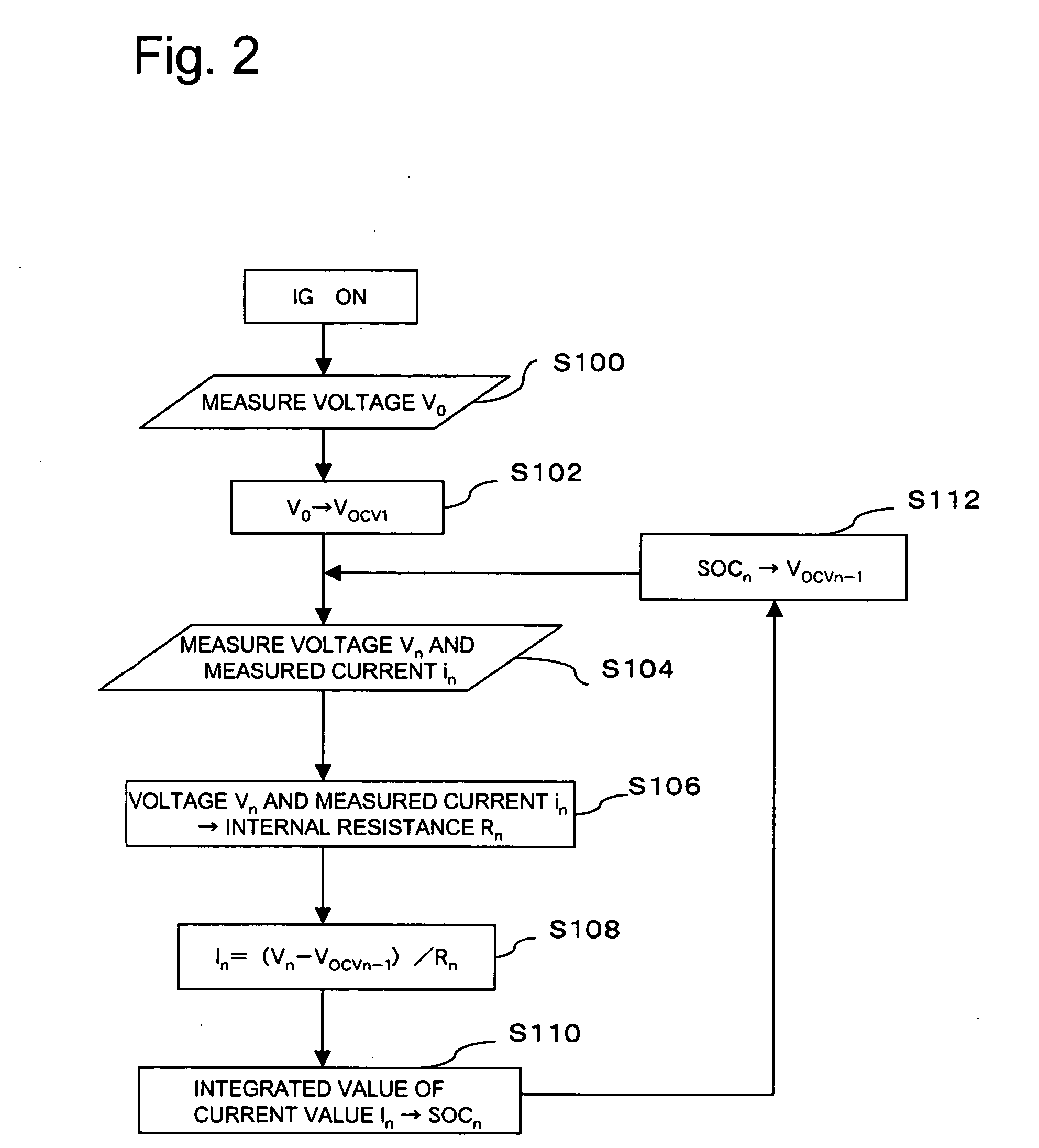
A battery ECU estimates the SOC by integrating the battery current measured by a current sensor, and the battery voltage Vn is measured by a voltage sensor and the battery temperature Tn is measured by a thermometer if the fluctuation of the charging / discharging current is great. If the number m of estimations of SOCn is m<10, m is incremented. The battery internal resistance Rn is estimated from the measured battery temperature Tn by using a correlation map showing the correlation between the previously stored battery temperature T and the battery internal resistance R. An estimation charging / discharging current In is determined using the measured battery voltage Vn, the battery open voltage Vocvn−1 determined on the basis of the previously estimated charged state, and the estimated battery internal resistance Rn. The SOCn is estimated by integrating the estimated charging / discharging current In. If the number m of estimations of the SOCn is m=10, the number m of estimations is changed to 0. The charging / discharging current in is measured by a current sensor. The battery internal resistance Rn is calculated from the battery voltage Vn and the charging / discharging current in. The battery temperature Tn is also measured, and the T-R correlation map is corrected.
Owner:TOYOTA JIDOSHA KK
Simple optimal estimator for PbA state of charge
InactiveUS20050046388A1Batteries circuit arrangementsCurrent/voltage measurementBattery state of chargeTerminal voltage
A state of charge estimator for a battery includes a meter that generates a terminal voltage signal of the battery and a terminal current signal of the battery. A controller employs a linearized model of the battery and a time-varying state estimator. The controller process a synthesized input based on the terminal current and the terminal voltage to estimate the battery state of charge.
Owner:GM GLOBAL TECH OPERATIONS LLC
Method and apparatus for online determination of battery state of charge and state of health
An online method and apparatus for determining state of charge (SoC) and state of health (SoH) of batteries on platforms that present dynamic charge and discharge environments is disclosed. A rested open circuit voltage (OCV) may be estimated online using a battery dynamic model along with measured terminal voltage, current and temperature. The SoC and SoH can then be determined from this estimated OCV. The apparatus and methods may estimate SoC and SoH of a battery in a real-time fashion without the need to a) disconnect the battery system from service; b) wait for a predefined rest time; or c) depolarize the battery.
Owner:HONEYWELL INT INC
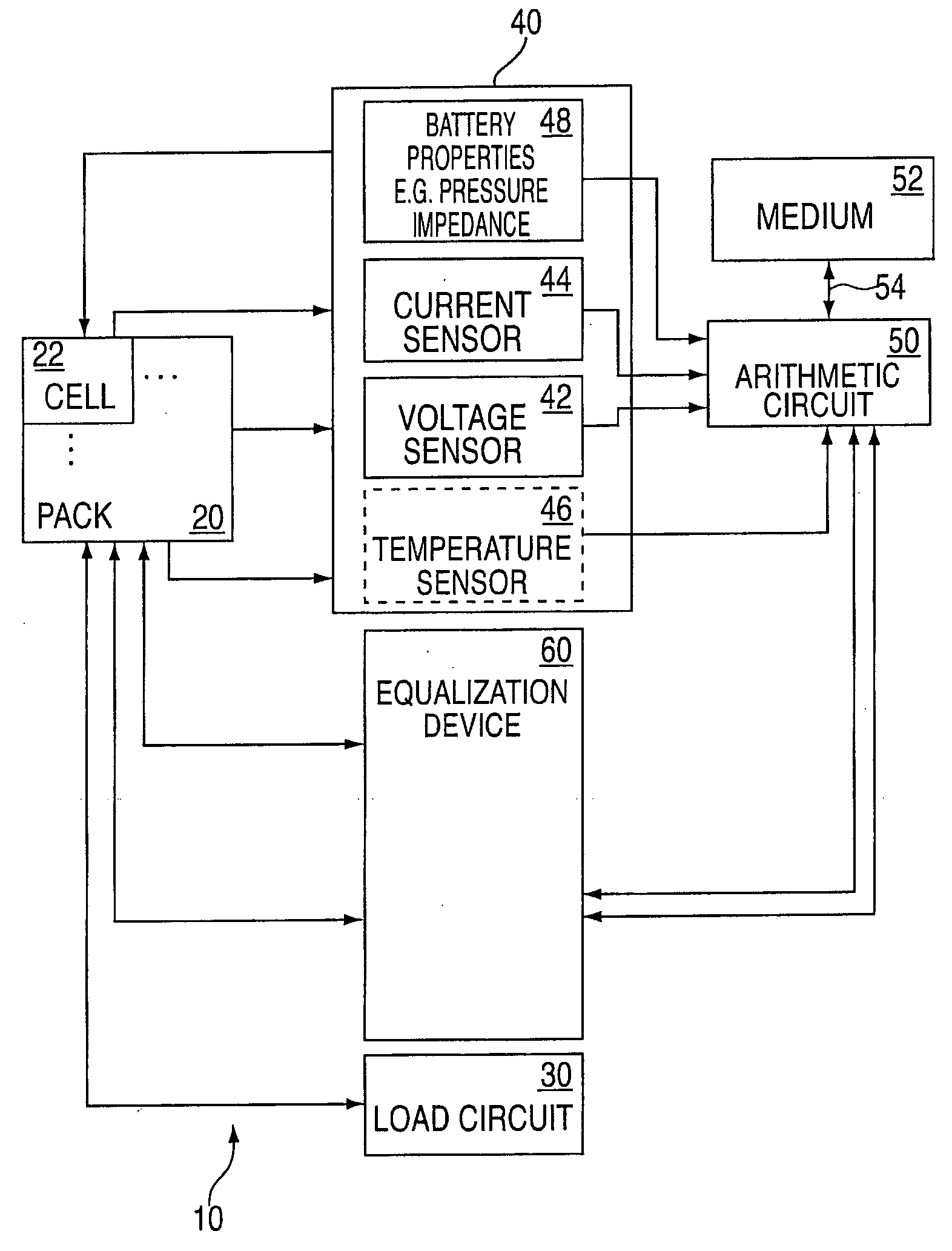
Method and system for cell equalization using state of charge
ActiveUS7525285B2Charge equalisation circuitCircuit monitoring/indicationBattery state of chargeKaiman filter
The present invention relates to an implementation of a method for cell equalization. The present invention uses other information such as the individual cell state-of-charge (SOC) estimates, and individual capacities and / or cell Coulombic efficiencies, possibly available from a dual extended Kalman filter. The present invention defines or refers to an operational SOC range between a minimum and a maximum. The minimum and maximum could be constants or could be dynamic based on functions of other variables. SOC can be measured relative to maximum charge or relative to maximum discharge for each cell. When the SOC is not consistent across the cells, a prioritizing scheme is used to determine which cells require equalization.
Owner:LG ENERGY SOLUTION LTD
Method for measuring state of charge of battery
InactiveCN101359036AAccurate state of charge SOC valueImprove performanceElectric devicesElectrical testingBattery state of chargeEngineering
A method for determining the battery state of charge SOC adopts corrected ampere hour integration method (Figure) to determine the battery state of charge SOC value at the time of t; the measurement method of the correction function fai (t) comprises the following steps: an SOC theoretical value calculated formula is adopted to calculate the battery state of charge SOC theoretical values SOC(theoretical) at a plurality of times; and the battery state of charge SOC actual values SOC(actual) at the plurality of times are measured; through the least square method, the correction function fai (t) standing for the relation between the difference value between the SOC(theoretical) and the SOC(actual), and the plurality of times; wherein, the SOC theoretical value calculation formula in step (a) adopts the basic ampere hour integration method (see the above formula); and x stands for one of the plurality of times. The application of the method can obtain accurate correction functions so as to achieve more precise battery state of charge SOC values to give full play to the battery performance and ensure the safety of the complete vehicle systems.
Owner:BYD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com