Methods of use of FGF homologs
a technology of fgf and homologs, applied in the field of methods of using fgf homologs, can solve the problems of affecting the function of the heart, unable to achieve normal cardiac hyperplasia mechanisms, and limited current treatment options for preventing tissue damage resulting from strok
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Extension of EST Sequence
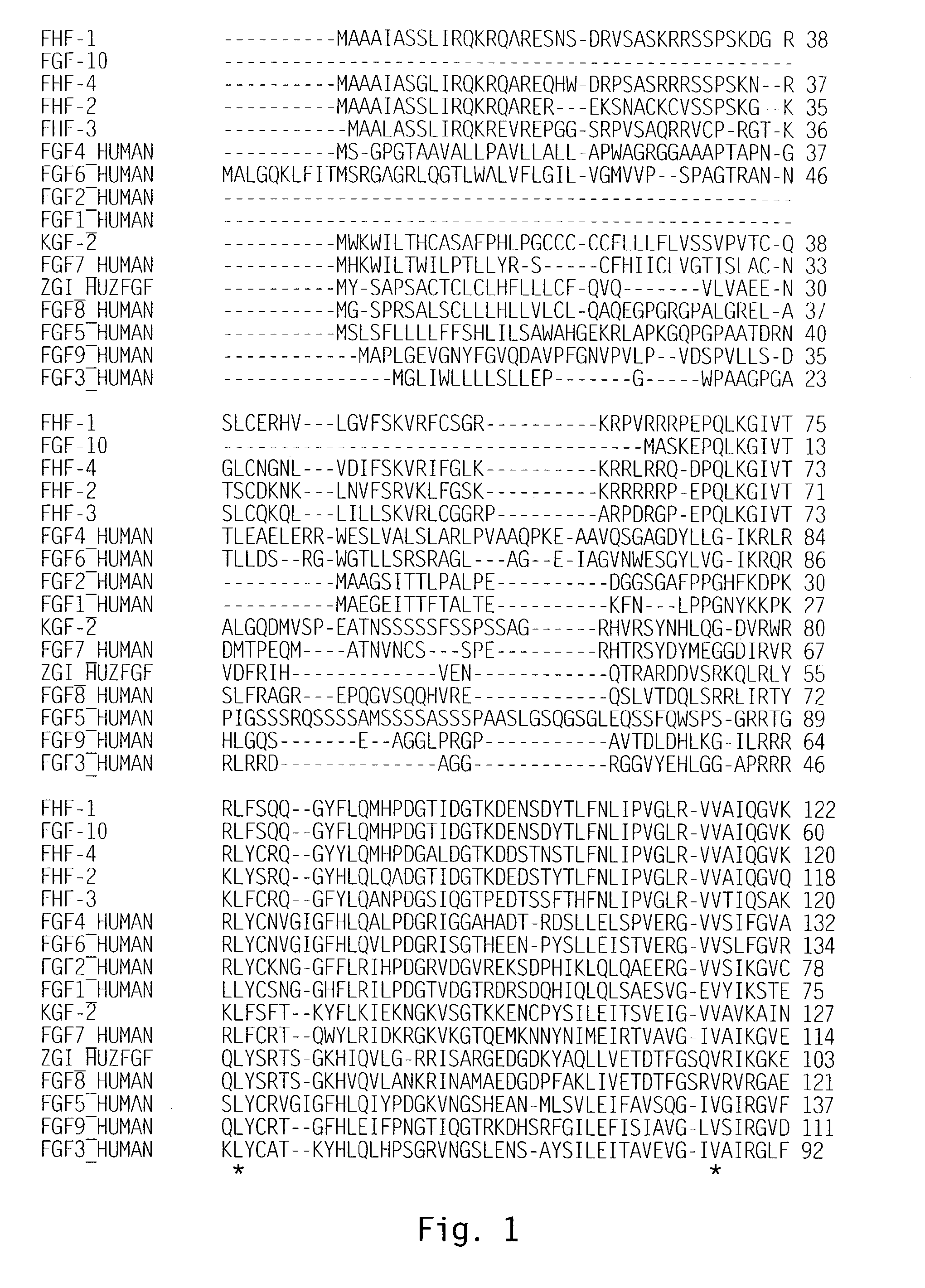
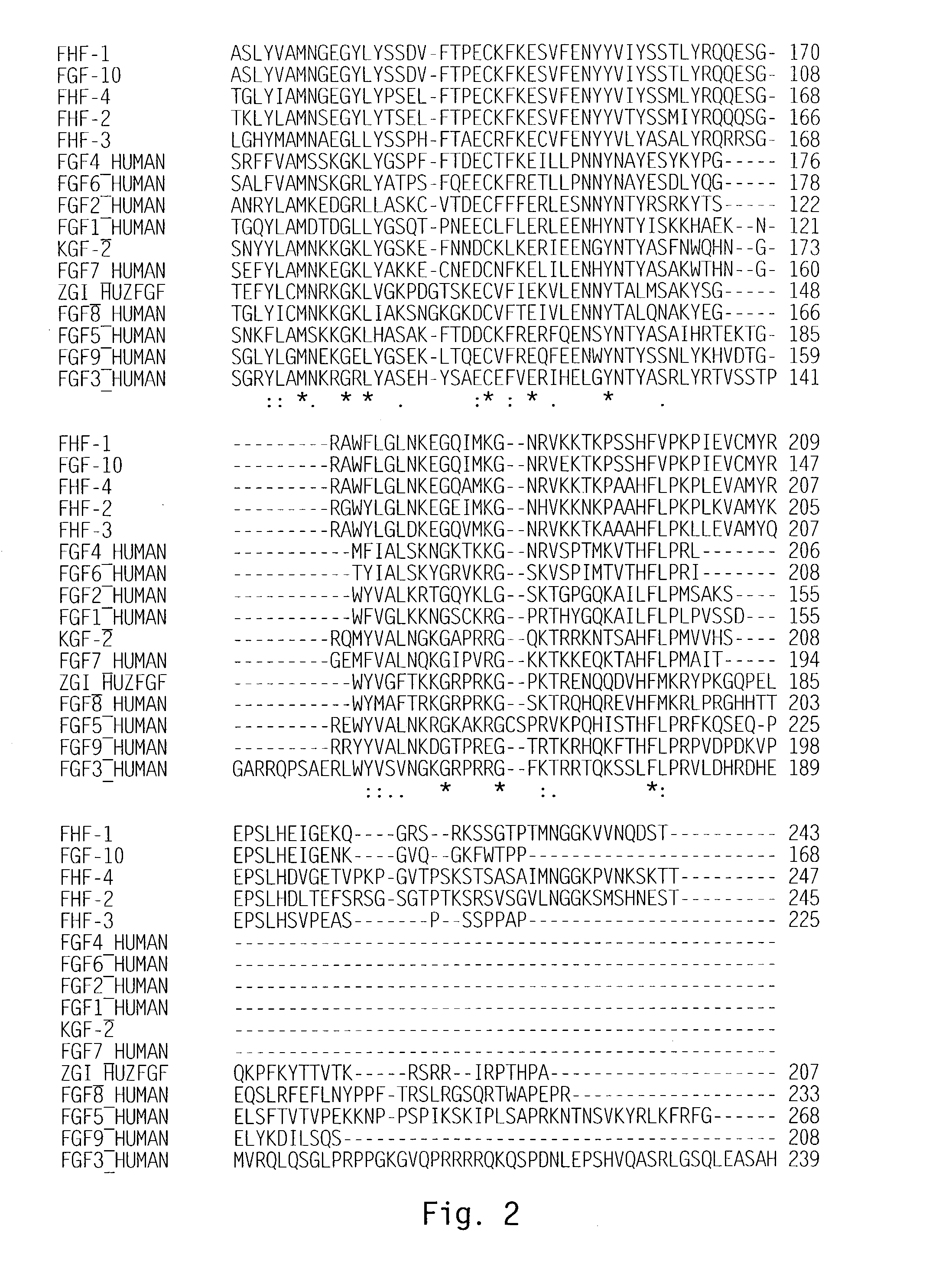
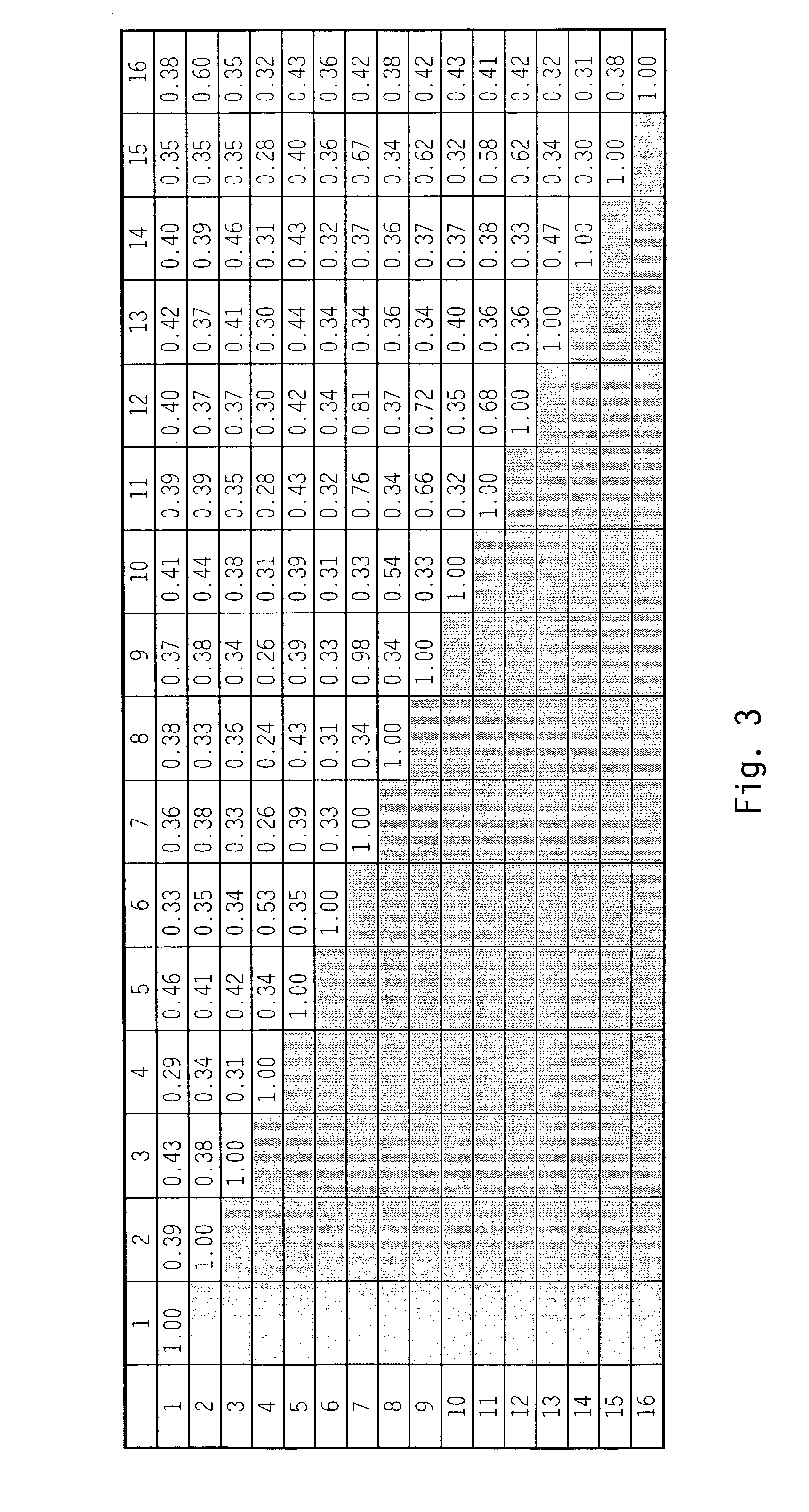
[0143]Scanning of a translated DNA database using a query for growth factors resulted in identification of an expressed sequence tag (EST) sequence found to be a novel member of the FGF family, and designated zFGF5.
[0144]Oligonucleotide primers ZC11676 (SEQ ID NO: 3) and ZC11677 (SEQ ID NO: 4) were designed from the sequence of an expressed sequence tag (EST). The primers were used for priming internally within the EST, and when PCR was performed using MARATHON READY cDNA® (Clontech, Palo Alto, Calif.) from adult heart tissue as template in polymerase chain reaction (PCR).
[0145]The conditions used for PCR were 1 cycle at 94° C. for 90 seconds, 35 cycles at 94° C. for 15 seconds; 68° C. for 1 minute; followed by 1 cycle for 10 minutes at 72° C. and 4° C. incubation period. The PCR reaction recreated 160 bp of the EST sequence, and confirmed that EST sequence was correct.
[0146]Other libraries that could be amplified with the oligonucleotide primers included sk...
example 2
[0147]Northerns were performed using Human Multiple Tissue Blots from Clontech (Palo Alto, Calif.). The 160 bp DNA fragment described in Example 1 was electrophoresed on a 1% agarose gel, the fragment was electroeluted, and then radioactively labeled using a random priming MEGAPRIME DNA® labeling system (Amersham, Arlington Heights, Ill.) according to the manufacturer's specifications. The probe was purified using a NUCTRAP® push column (Stratagene Cloning Systems, La Jolla, Calif.). EXPRESSHYB® (Clontech, Palo Alto, Calif.) solution was used for prehybridization and as a hybridrizing solution for the Northern blots. Hybridization took place overnight at 8° C., and the blots were then washed in 2×SSC and 0.05% SDS at RT, followed by a wash in 0.1×SSC and 0.1% SDS at 50° C. A single band was observed at approximately 2.0 kb. Signal intensity was highest for adult heart with relatively less intense signals in skeletal muscle and stomach. Dot blots were probed essent...
example 3
Assay for In Vitro Activity of zFGF5
A.
[0148]The mitogenic activity of zFGF5 is assayed using cell lines and cells from a primary culture. Conditioned medium from cells expressing the recombinant protein and / or purified protein is added to cultures of the following cell lines: NIH 3T3 fibroblast (ATCC No. CRL-1658), CHH-1 chum heart cells (ATCC No. CRL-1680), H9c2 rat heart myoblasts (ATCC No. CRL-1446), Shionogi mammary carcinoma cells (Tanaka et al., 1992, ibid.) and LNCaP.FGC adenocarcinoma cells. Freshly isolated cells useful for testing the proliferative activity of zFGF5 include: cardiac fibroblasts, cardiac myocytes, skeletal myocytes and human umbilical vein endothelial cells.
[0149]Mitogenic activity is assayed by measurement of 3H-thymidine incorporation based on the method of Raines and Ross (Meth. Enzymology 109:749–773, 1985). Briefly, quiescent cells are plated cells at a density of 3×104 cells / ml in an appropriate medium. A typical growth medium is Dulbecco's Growth Med...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| time constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com