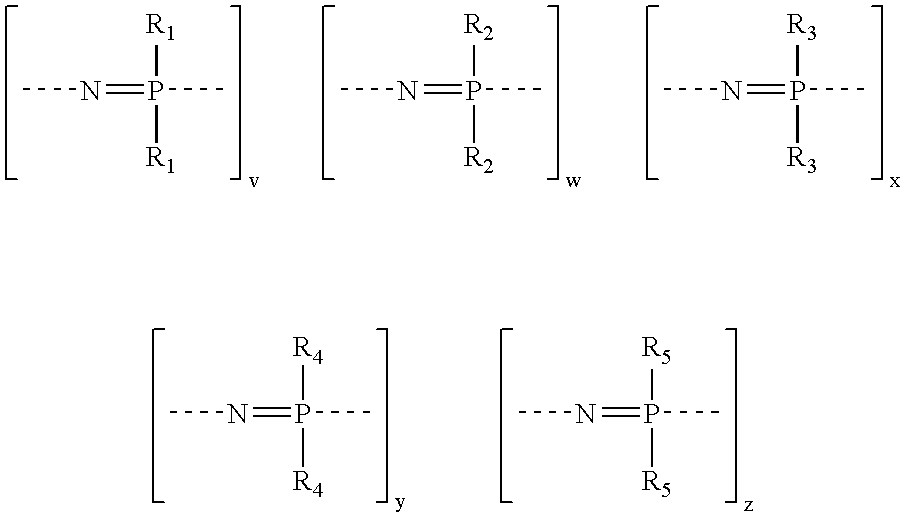
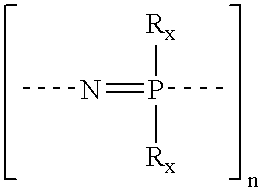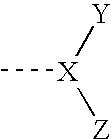Sulfonated polyphosphazenes, uses thereof, and methods for preparing same
a technology of sulfonated polyphosphazene and polydichlorophosphazene, which is applied in the field of sulfonated polyphosphazene, uses of sulfonated polyphosphazene, and methods for preparing the same. it can solve the problems of significant decrease in the yield and molecular weight of the final polymer product, polydichlorophosphazene with sodium salts of sul
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0039]4-hydroxybenzenesulfonic acid, sodium salt dehydrate (4.57 g, 19.7 mmole) is dissolved in water (100 mL) and is titrated with a NaOH solution (19.7 ml, 1 N). The resultant 4-hydroxybenzenesulfonic acid, disodium salt is lyophilized.
[0040]4-hydroxybenzenesulfonic acid, disodium salt (0.50 g, 2.12 mmol)) benzyltriethylammonium bromide (0.58 g, 2.12 mmol), sodium phenoxide (3.61 ml of a 12.3% w / v solution in diglyme, 3.94 mmol), chlorobenzene (40 mL), and DMF (4 ml) are placed in a 100 CC Telfon™ reaction tube fitted with a magnetic stir bar. Under a stream of nitrogen the reaction mixture is heated to 105° C. and agitated for 30 minutes after which time, a solution of polydichlorophosphazene in diglyme (2.00 mL of a 9.6% solution w / v, 0.83 mmole) is added. The reaction mixture is heated at 120° C. and agitated for two hours. The resultant polymer is precipitated with hexane and dried under vacuum. The yield was 0.47 g. The polymer was characterized by gel permeation chromatograp...
example 3
[0041]4-hydroxybenzenesulfonic acid, sodium salt dehydrate (4.57 g, 19.7 mmole) is dissolved in water (100 mL) and is titrated with a NaOH solution (˜19.7 mL, 1 N). The resultant 4-hydroxybenzenesulfonic acid, disodium salt is lyophilized.
[0042]4-hydroxybenzenesulfonic acid, disodium salt (0.36 g, 1.52 mmol)) benzyltriethylammonium bromide (0.41 g, 1.52 mmol), sodium phenoxide (4.55 ml of a 12.3% w / v solution in diglyme, 4.16 mmol), chlorobenzene (40 mL), and DMF (4 ml) are placed in a 100 CC Telfon™ reaction tube fitted with a magnetic stir bar. Under a stream of nitrogen the reaction mixture is heated to 105° C. and agitated for 30 minutes after which time, a solution of polydichlorophosphazene in diglyme (2.00 mL of a 9.6% solution w / v, 0.83 mmole) is added. The reaction mixture is heated at 120° C. and agitated for two hours. The resultant polymer is precipitated with hexane and dried under vacuum. The yield was 0.42 g. The polymer was characterized by gel permeation chromatogra...
example 4
[0043]4-hydroxybenzenesulfonic acid, sodium salt dehydrate (4.57 g, 19.7 mmole) is dissolved in water (100 mL) and is titrated with a NaOH solution (˜19.7 mL, 1 N). Benzyltriethylammonium bromide (5.36 g, 19.7 mmole) is added to this mixture and the resultant product is lyophilized, yielding a white powder of 4-hydroxybenzenesulfonic acid, dibenzyltriethylammonium.
[0044]4-hydroxybenzenesulfonic acid, dibenzyltriethylammonium (5.73 g, 8.45 mmol), diglyme (40 mL), and DMF (5 mL) are placed in a 100 CC Teflon™ reaction tube fitted with a magnetic stir bar. Under a stream of nitrogen the reaction mixture is heated to 120° C. and agitated for two hours after which time, a solution of polydichlorophosphazene in diglyme (0.33 mL of a 15% solution w / v, 0.4 mmole) is added. The resultant heterogeneous mixture is heated at 120° C. and agitated for two hours. The solution is cooled to 80° C. and the organic layer is removed. The precipitate is dissolved in water and purified by dialysis agains...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com