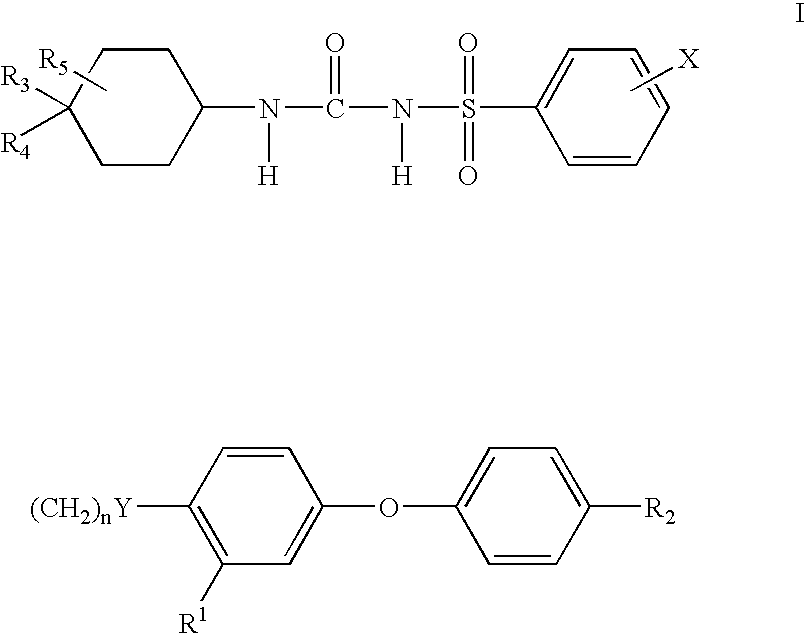
N-cyclohexylaminocarbonyl benzensulfonmide derivatives
a technology of n-cyclohexylaminocarbonyl benzenesulfonamide and derivatives, which is applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of increased and premature morbidity and mortality, elevated plasma insulin levels are not sufficient to overcome the pronounced insulin resistance, and the serum glucose level is not elevated enough to meet the criteria of type 2 diabetes, etc., to improve the insulin resistance of non-
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
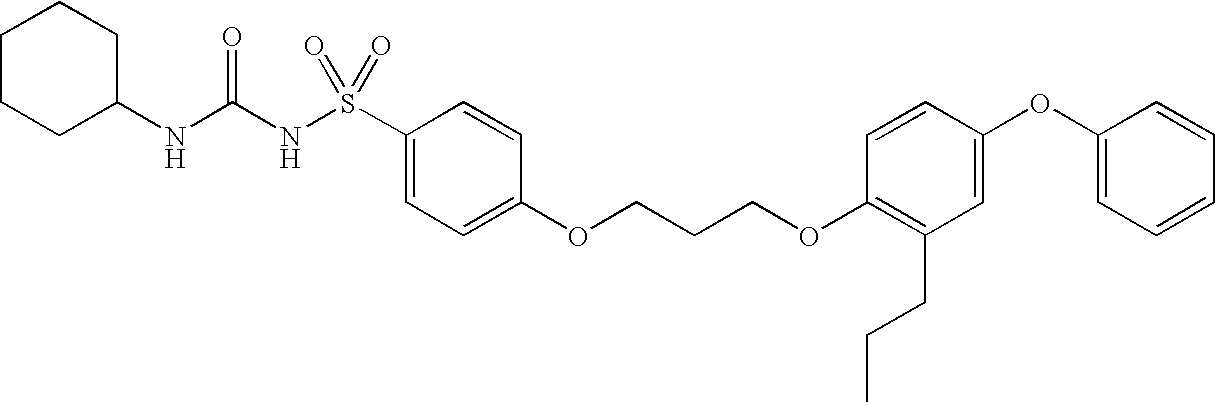
example 1
N-[(cyclohexylamino)carbonyl]-4-[3-(4-phenoxy-2-propylphenoxy)propoxy]benzenesulfonamide
[0088]
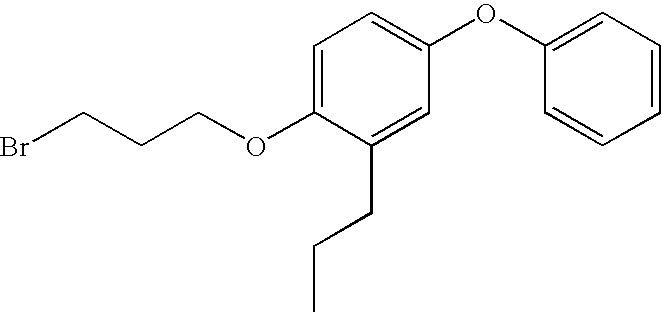
Step A: Preparation of 1-(3-bromopropoxy)4-phenoxy-2-propylbenzene
[0089]
[0090]The title compound was prepared according to the method described in U.S. Pat. No. 6,008,237, Example 11, Step A.
Step B: Preparation of 4-[3-(4-phenoxy-2-propylphenoxy)propoxy]benzenesulfonamide
[0091]
[0092]To a solution of 1-(3-bromopropoxy)-4-phenoxy-2-propylbenzene (1.01 g, 2.9 mmol) and 4-hydroxybenzenesulfonamide (0.5 g, 2.9 mmol) in dry DMF (29 mL) was added cesium carbonate (1.04 g, 3.2 mmol). The resulting suspension was stirred in a 50° C. oil bath for 5 h. After cooling to room temperature, the reaction suspension was concentrated under vacuum to a residue, which was then partitioned between ethyl acetate and water. The ethyl acetate phase was dried over sodium sulfate, filtered, and concentrated to an oil. The oil was chromatographed over silica gel with hexanes / ethyl acetate (2:1) to isolate the title c...
example 2
N-[(cyclohexylamino)carbonyl]-3-[3-(4-phenoxy-2-propylphenoxy)propoxy]benzenesulfonamide
[0095]
Step A: Preparation of 3-hydroxybenzenesulfonamide
[0096]
[0097]To a solution of 3-aminobenzenesulfonamide (2.35 g, 13.6 mmol) in 30% sulfuric acid (18 mL) stirred in a 0° C. ice-water bath was added dropwise a solution of sodium nitrite (1.22 g, 17.7 mmol) in water (10 mL). The resulting reaction solution continued to stir in an ice-water bath for 30 min. The solution was then stirred in a 100° C. oil bath for 30 min. After cooling to room temperature, the reaction solution was partitioned between ethyl acetate and brine. After shaking, the aqueous phase was extracted with ethyl acetate. The combined ethyl acetate phases were dried over sodium sulfate, filtered, and concentrated to a yellow solid (2.11 g, 89% yield).
Step B: Preparation of 1-(3-bromopropoxy)-4-phenoxy-2-propylbenzene
[0098]
[0099]The title compound was prepared according to the method described in U.S. Pat. No. 6,008,237 Exampl...
examples 3-9
[0104]The compounds written below as Examples 3-9 were made using the methodology that was described in Examples 1 and 2, starting with chemical compounds that are readily made or readily available. The syntheses are readily accomplished by one of ordinary skill in the art.
Ex 3
N-[(cyclohexylamino)carbonyl]-4-{3-[4-(4-fluorophenoxy)-2-propylphenoxy]propoxy}benzenesulfonamide
[0105]
Ex 4
4-{3-[2-chloro-4-(4-fluorophenoxy)phenoxy]propoxy}-N-[(cyclohexylamino)carbonyl]benzenesulfonamide
[0106]
Ex 5
N-[(cyclohexylamino)carbonyl]-4-(3-{4-[4-(methylsulfonyl)phenoxy]-2-propylphenoxy}propoxy)benzenesulfonamide
[0107]
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com