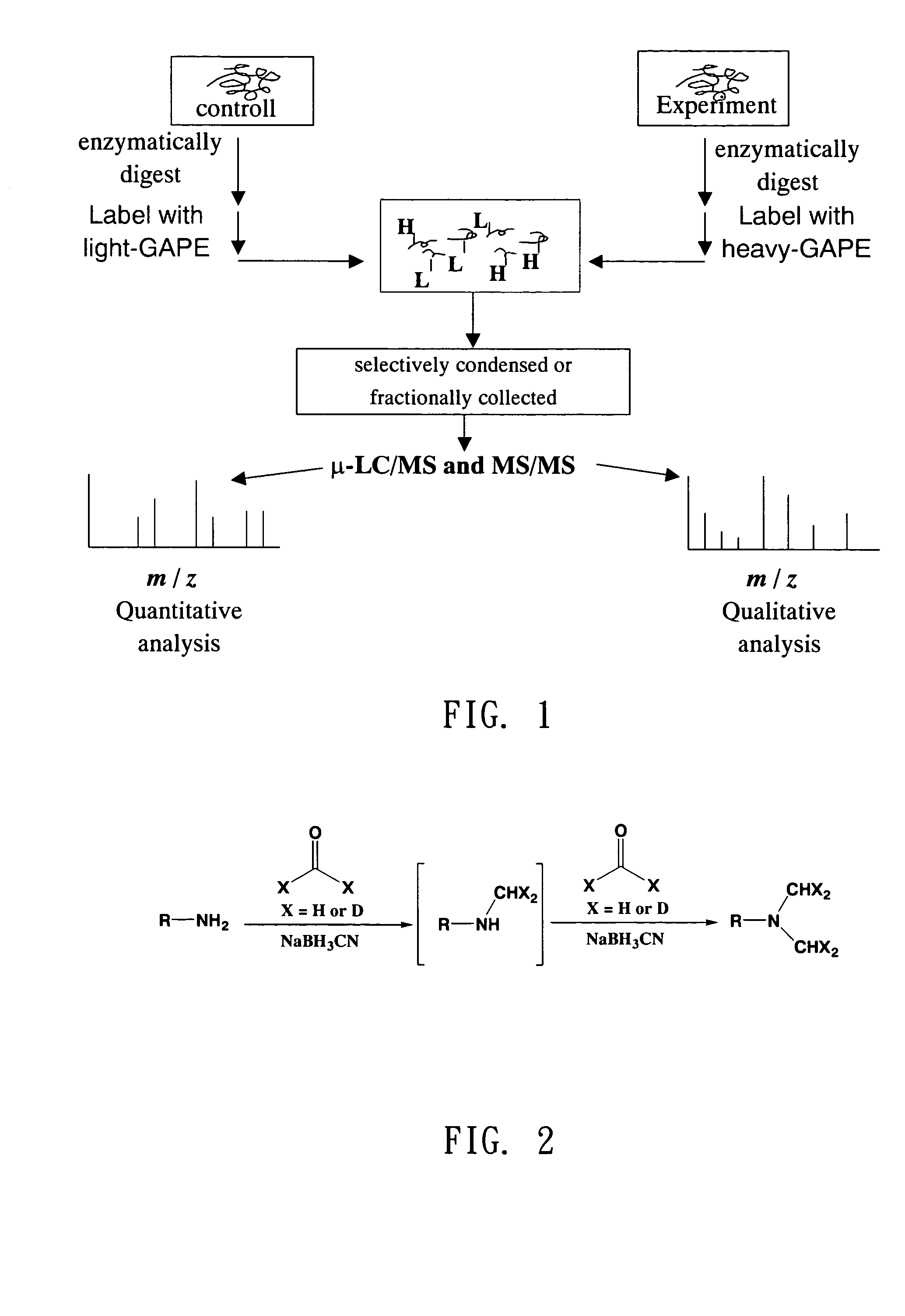
Reagent kit of global analysis for protein expression and method for qualitative and quantitative proteomic analysis using the same
a global analysis and protein technology, applied in the direction of material analysis using wave/particle radiation, instruments, peptides, etc., can solve the problems of inability to cover all types of protein expression, inability to reproduce or accurately, and limited methods, etc., to achieve accurate, fast and economical platforms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Effect of GAPE Reagent Kit on the Mass Spectrometric Signals of Peptides
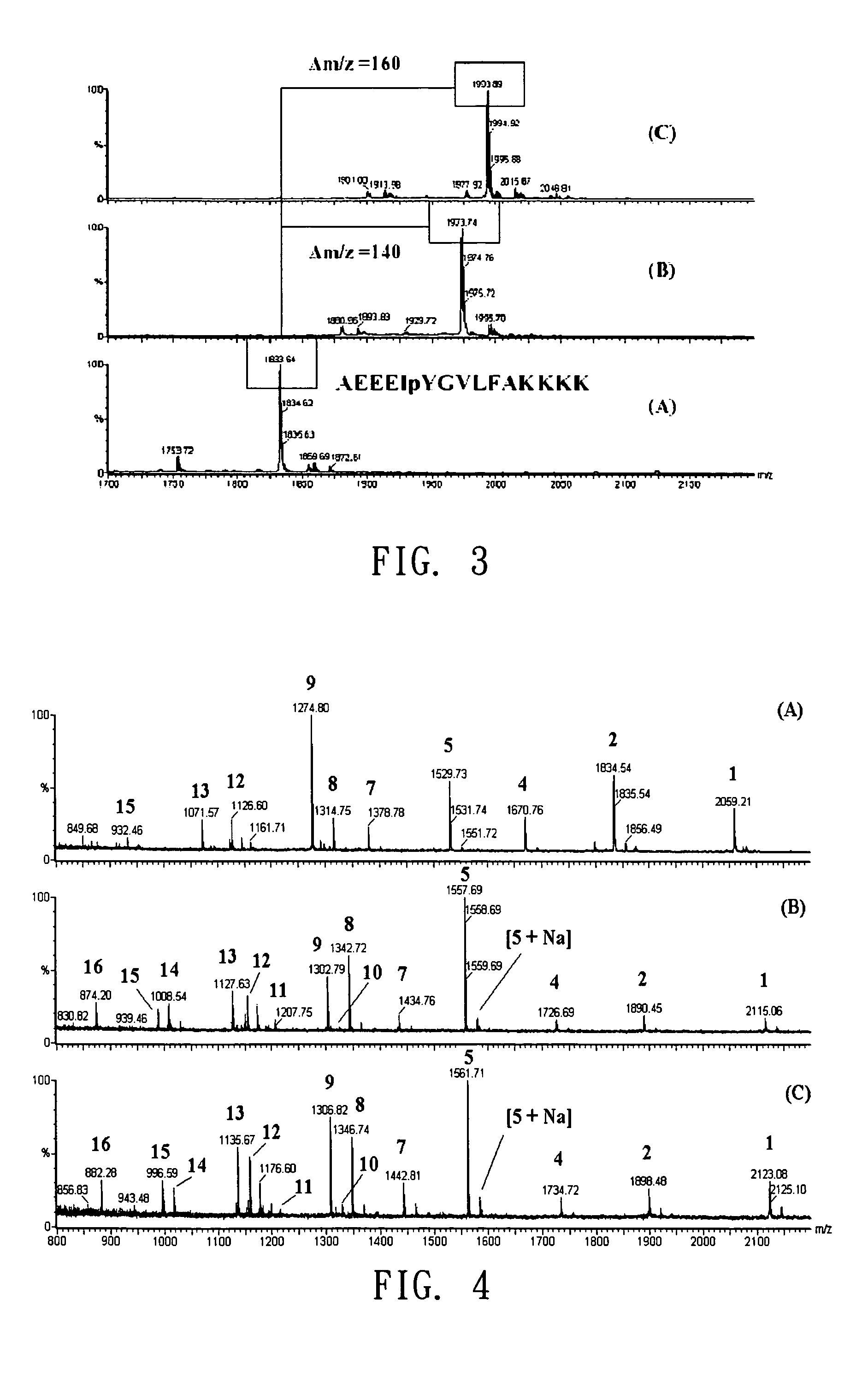
[0047]Label the standards of phosphopeptides (SEQ:AEEElpYGVLFAKKK) with GAPE reagent kit containing formaldehyde (light-GAPE) and doubly deuterated formaldehyde (heavy-GAPE). The aldehyde compound, under the aid of reducing agent, would react with the N-terminal amino and ε-amino of lysine in the peptide to form dimethyl amine. The reaction takes only 5 minutes and the labeling mechanism is as shown in FIG. 2. Subsequently analyze the labeled samples using MALDI-MS and the results are shown in FIG. 3, in which (A) is the mass spectrogram of phosphopeptide standard not labeled with GAPE reagent kit; (B) is the mass spectrogram of light-GAPE labeled phosphopeptide standard; and (C) is the mass spectrogram of heavy-GAPE labeled phosphopeptide standard. As shown, GAPE reagent kit can specifically label N-terminal amino group and the amino group of lysine in the peptides, and the molecular weight difference betwe...
example 2
The Effect of GAPE Reagent Kit on Retention Time in Liquid Chromatography
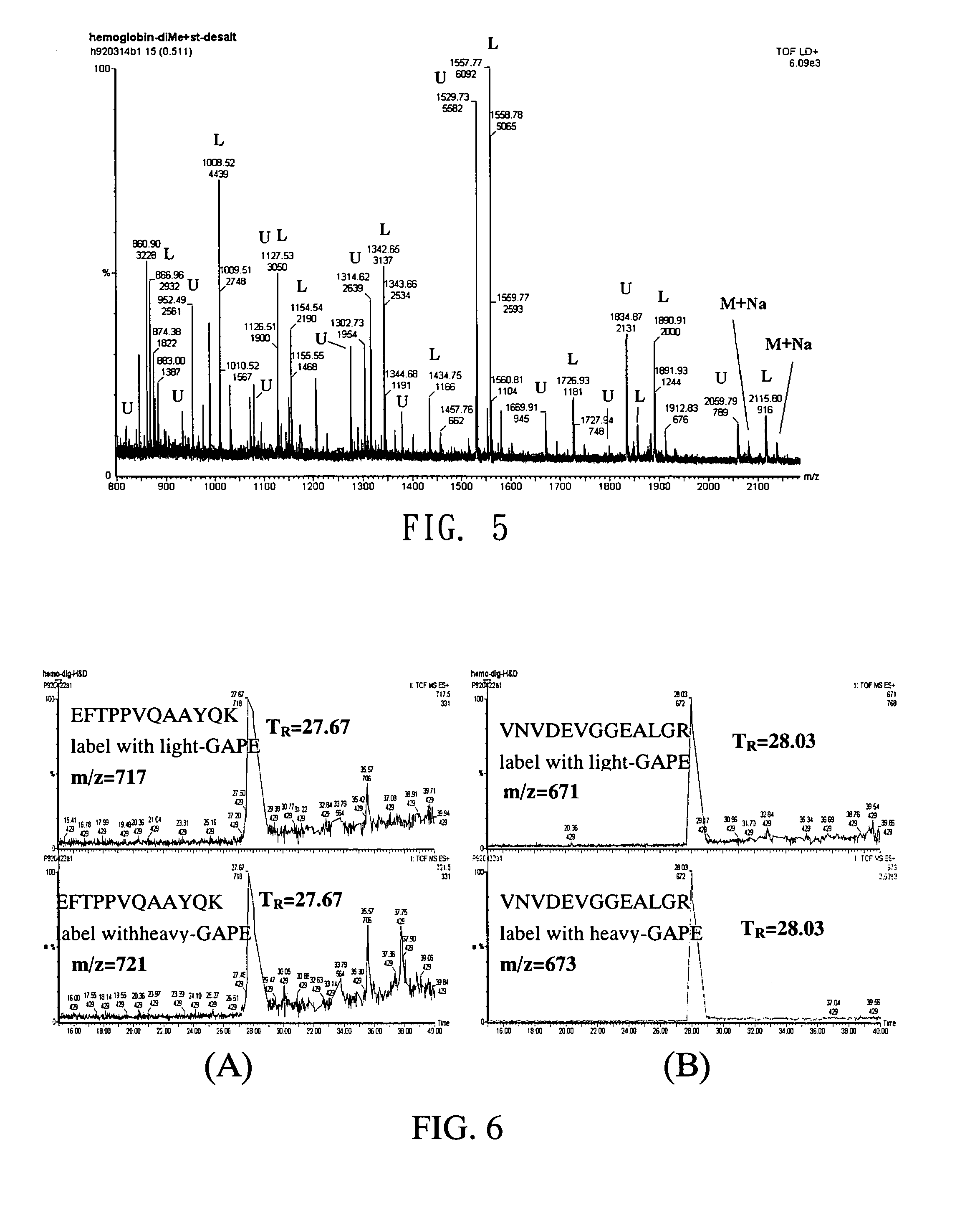
[0050]Provide two equivalent hemoglobin digest samples and label them with light-GAPE and heavy-GAPE respectively. Mixed the two labeled samples and analyze with liquid chromatography-mass spectrometry (LC-MS). The results are shown in FIG. 6. FIG. 6(A) shows the retention time of peptides (SEQ:EFTPPVQAAYQK), which is 27.67 minutes for both light-GAPE and heavy-GAPE modified peptides; FIG. 6(B) shows the retention time of peptides (SEQ:VNVDEVGGEALGR), which is 28.03 minutes for both light-GAPE and heavy-GAPE modified peptides. In summary of the results in FIG. 6, the light-GAPE and heavy-GAPE modified peptides have the same retention time in LC, which provides an ideal condition for subsequent MC analyses.
example 3
Detection of Relative Protein Expression Levels Using GAPE Reagent Kit
[0051]Label phosphopeptide standards (SEQ:AEEElpYGVLFAKKK) with light-GAPE and heavy-GAPE, and then mix the labeled samples in different ratios (2:1, 1:1, 1:1.5, 1:2, 1:5, 1:20). Analyze the mixed samples using MALDI-MS. Based on the results as shown in FIG. 7, the molecular weight difference between light-GAPE and heavy-GAPE modified peptides in mass spectrometry is 4(n+1) and the relative intensity of MS signals are consistent with the mix ratio. FIG. 8 is the result of mix ratio plotted against relative intensity of MS signals, which shows excellent linear relationship (R2=0.9939), indicating that the GAPE reagent kit can accurately detect the relative protein expression levels.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| stable isotope labeling | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com