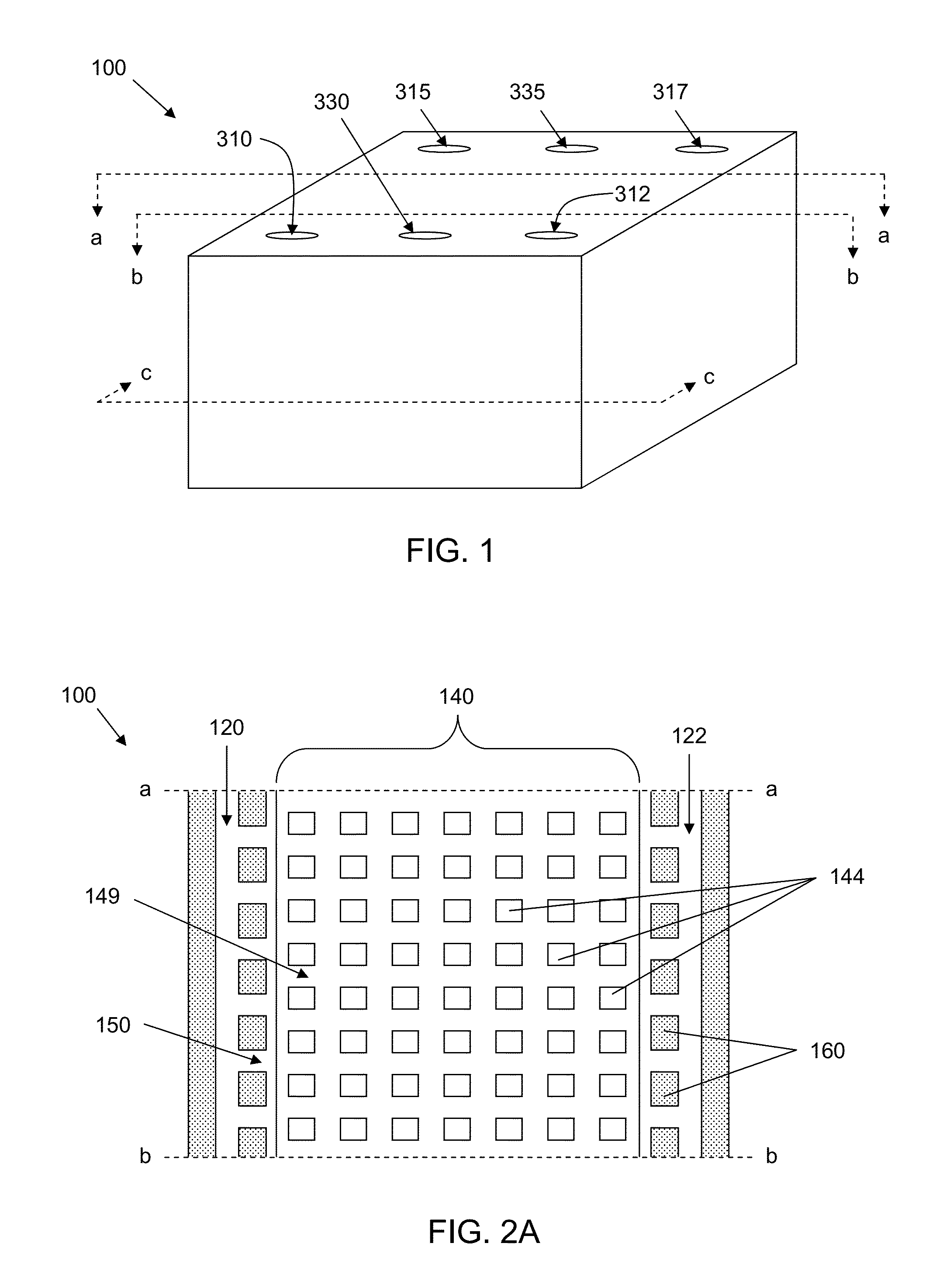
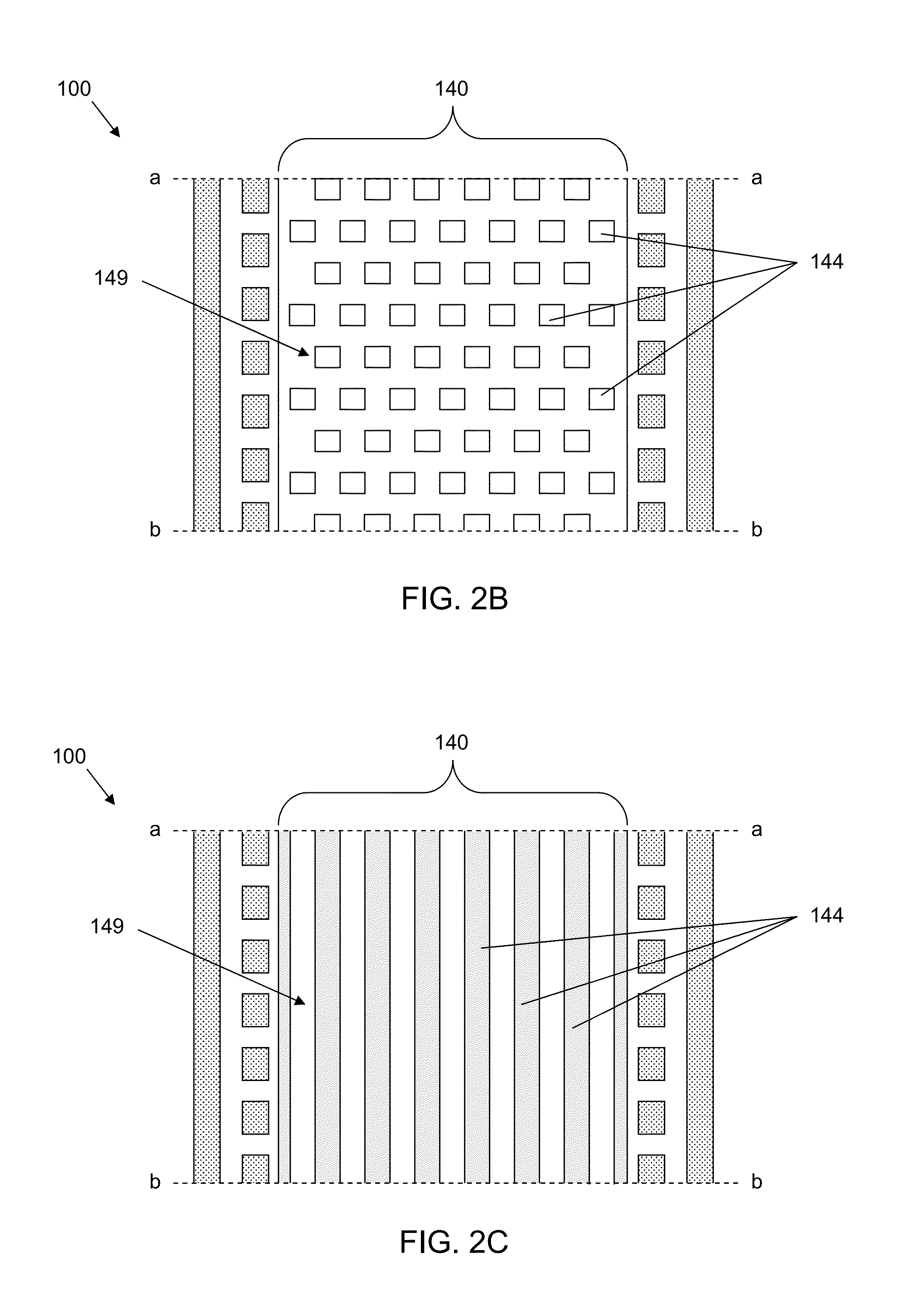
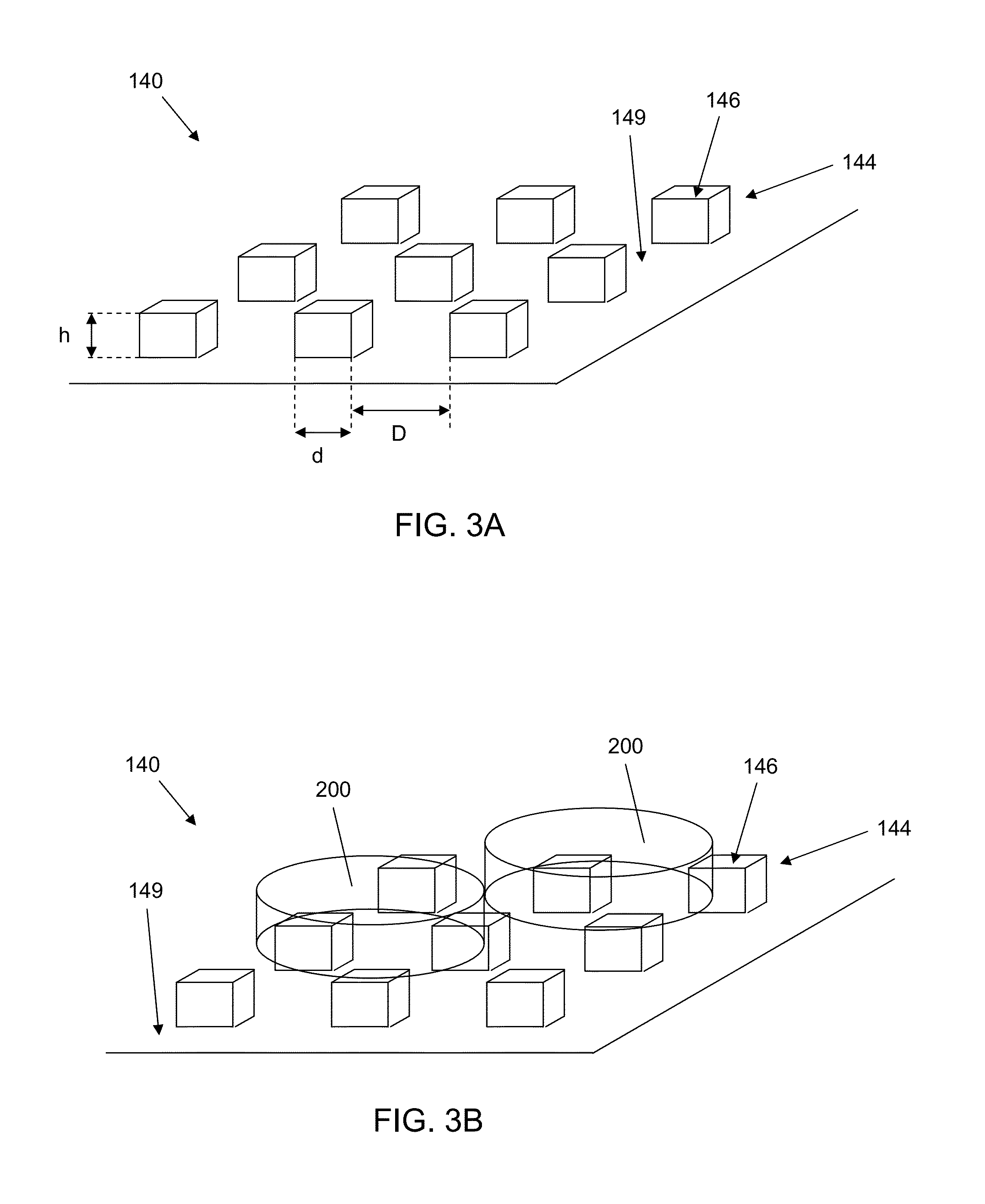
Microfluidic device for cell culture
a cell culture and microfluidic technology, applied in the field of microfluidic cell culture apparatuses, can solve the problems of limited improvement of cell cultured cell function, difficulty in mimicking complex in vivo microenvironment that modulates cellular function, and inability to achieve long-term cell culture success, etc., to promote cell polarity, promote efficient and effective transport, and encourage cell polarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Device Fabrication and Assembly
[0094]Four inch silicone wafers were primed with P-20 (Microprime Primer P-20, Shin-Etsu MicroSi, Phoenix, Ariz.), and a 1 um thick Shipley 1813 photoresist (Rohm and Haas, Philadelphia, Pa.) was spun on the wafer at 3000 rpm for 30 sec (acceleration 1000 rpm / s) and soft baked on a hot plate for 1 min at 110° C. The wafers were exposed to UV-light through a chromium mask with the desired structures designed as CAD-drawing using MA6 (Karl Suss) mask aligner. After post bake of 2 min at 80° C. the wafers were finally developed (60-100 s, MF-319, Shipley), thoroughly rinsed with water and dried. Molds for 15 um deep troughs and 45 um deep fluidic channels and cell culture chamber were etched into the silicone using Plasma Therm 72 fluorine based reactive ion etcher. After photoresist stripping and cleaning silicone masters were exposed to trichloro(1H, 1H, 2H, 2H)-perfluorooctyl vapor for 2 h for passivation. Polydimethylsiloxane (PDMS) replicas were prod...
example 2
Fluidic Characterization of the Microfluidic Device
[0098]To test the mass transfer inside the device between perfusion channels and cell incubation chamber subsequent injections solutions of Sulforhodamine B (8.9×10−5 M SRB in PBS buffer) and carboxyfluoresceine (4×10−5 M in PBS buffer) dyes was performed employing a microfluidic device having a single inlet and outlet for both the left and right perfusion channels. An increase of Sulforhodamine B fluorescence intensity in the cell culture chamber was observed as a function of flow rate and time (FIG. 23A) indicating good fluidic transport across the retention barrier (posts) and between the perfusion channels and the cell chamber. Conversely, when the fluorescein (4×10−5 M in PBS buffer) was introduced via the cell chamber inlet, an increase in fluorescent intensity across the retention barrier (posts) and filling the perfusion channels was observed (FIG. 23B). In the images shown in FIGS. 23A and 23B, the left side of the image is...
example 3
Long Term Cell Culture in Microfluidic Devices
[0101]Incubation of human primary hepatocytes in the microfluidic devices was performed to demonstrate the ability of supporting phenotypically active cell population for prolonged periods of time. 5,000-10,000 primary human hepatocyte cells (Cryopreserved human hepatocytes, XenoTech, Lenexa, Kans.) were plated to the microfluidic devices (via cell chamber port) and the devices were perfused with MFE cell culture medium in open-loop mode at 1 ul / min flow rate. Cell culture media was manually changed daily in 96 well plates cultures. Devices were monitored daily to track possible changes in cell morphology and health. At different time points the incubation was stopped and a live / dead stain (LIVE / DEAD viability / cytotoxicity kit for mammalian cells from Molecular Probes, Eugene, Oreg.) was performed to monitor cell survival rate and morphology. Images of cells packed in the device and the results of live / dead stain are shown in FIGS. 25-26...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com