Electro-chemical deposition system and method of electroplating on substrates
a technology of electrochemical deposition and substrate, which is applied in the direction of electric circuits, manufacturing tools, coatings, etc., can solve the problems of aluminum also suffering from electromigration phenomena, many traditional deposition processes have difficulty filling sub-micron structures, and a great amount of ongoing effort being directed at the formation of void-free sub-micron structures, etc., to achieve the effect of easy maintenance of configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035]The present invention generally provides several embodiments of a new electrochemical cell and a method of operation of the cells to deposit high quality metal layers on substrates. The invention also provides new electrolyte solutions which can be used to advantage in the deposition of metals, and copper in particular, into very small features, i.e., micron sized features and smaller. The invention will be described below first in reference to the hardware, then operation of the hardware and then chemistry of the electrolyte solutions.
[0036]Electrochemical Cell Hardware
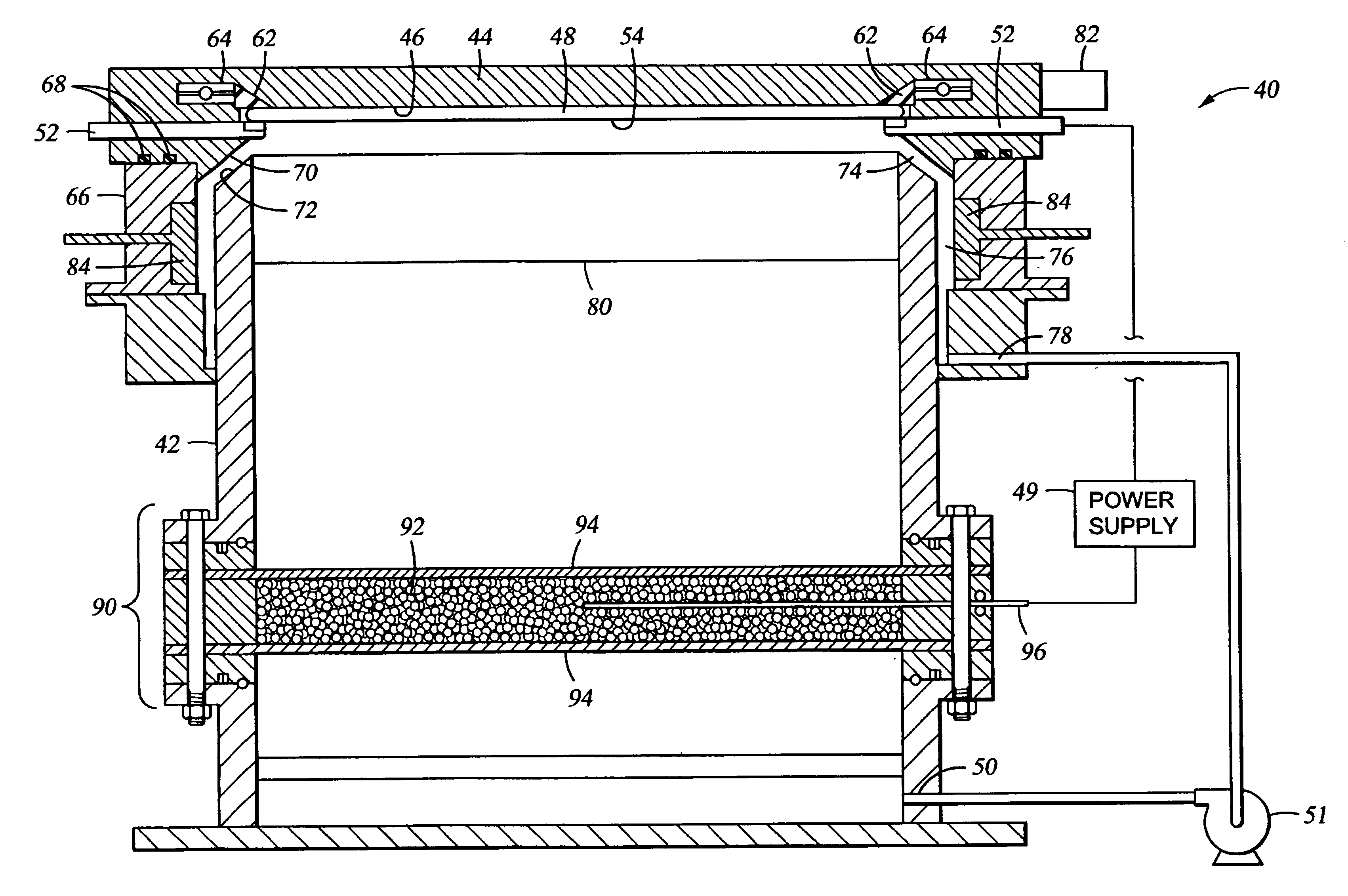
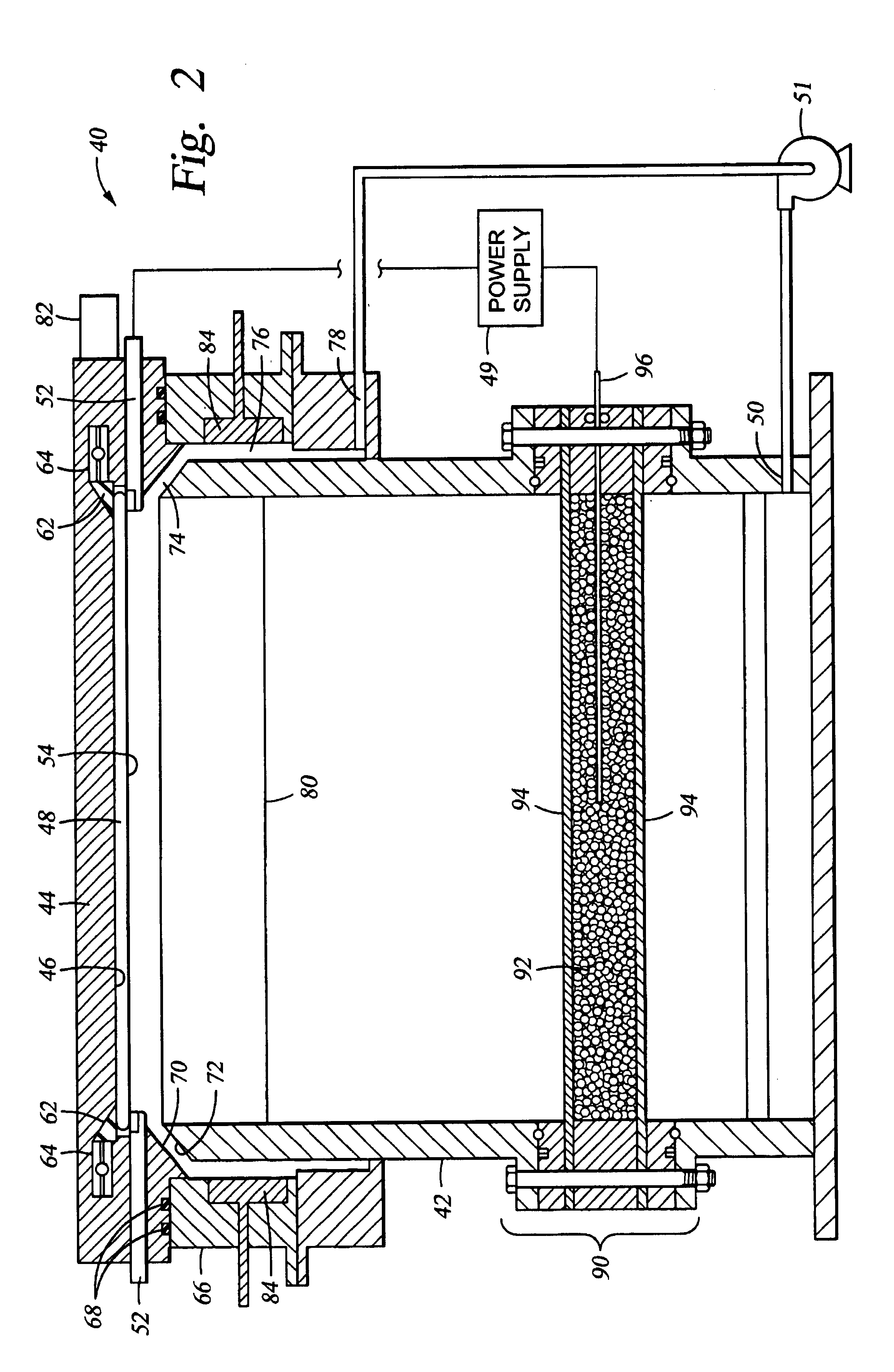
[0037]FIG. 2 is a cross sectional schematic view of a cell 40 for electroplating a metal onto a substrate. The electroplating cell 40 generally comprises a container body 42 having an opening on the top portion of the container body to receive and support a substrate holder 44 thereover. The container body 42 is preferably an annular cell comprised of an electrically insulative material, such a plastic, plexigl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| gap width | aaaaa | aaaaa |
| gap width | aaaaa | aaaaa |
| cathodic current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com