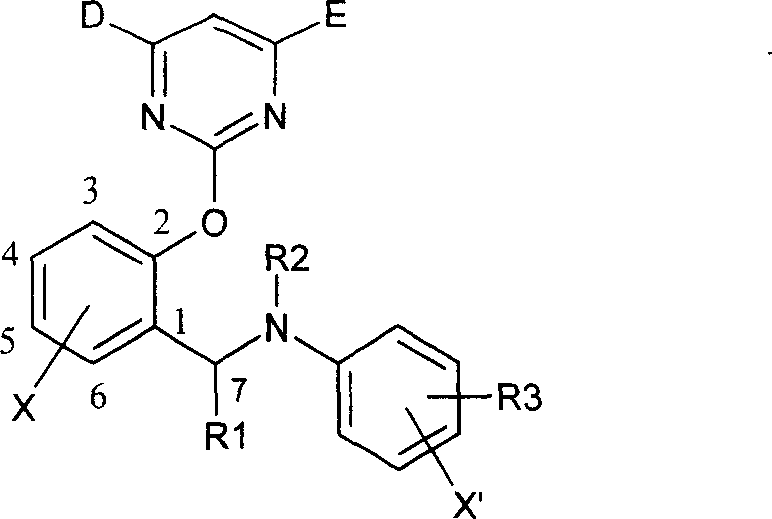
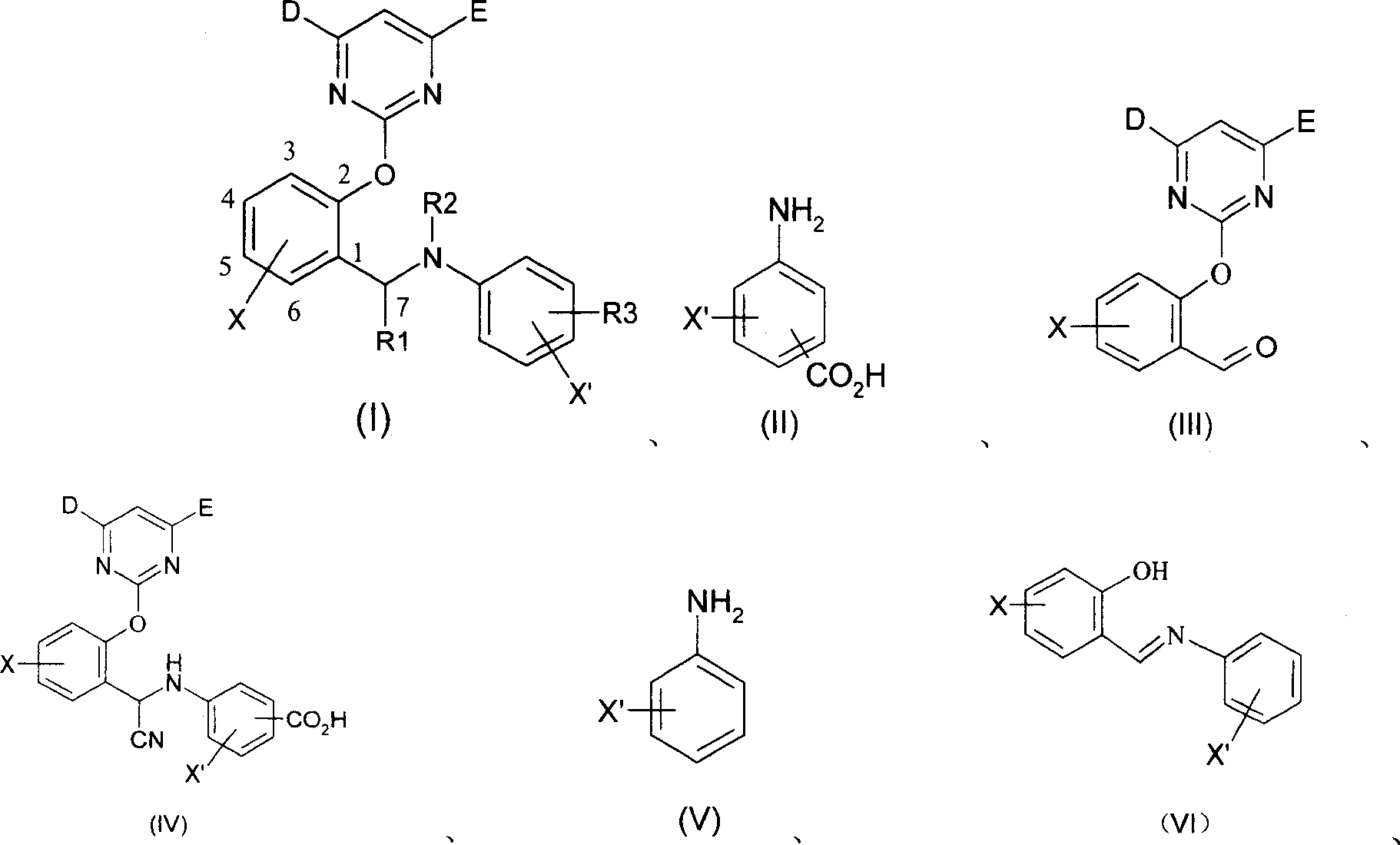
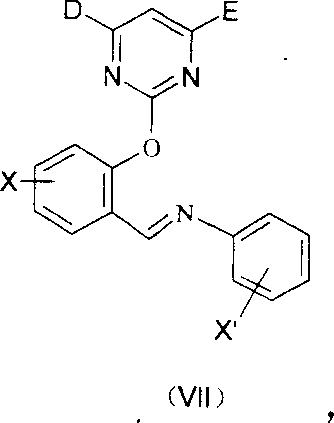
2-pyrimidine oxy-N-aryl 7-nitrile or organic phosphate benzylamine compound, its production and use thereof
A phosphate-based benzylamine, pyrimidineoxy technology, applied in the field of agrochemical herbicides, can solve problems such as limited new varieties of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthesis of 2-pyrimidinyloxy-N-aryl-7-cyanobenzylamine compound is example with (I-1):
[0059] The first step: 12.2 grams (0.1mol) salicylaldehyde, 21.8 grams (0.1mol) 4,6-dimethoxythiamphenicyl pyrimidine, 27.6 grams (0.2 mol) gram potassium carbonate and 150mL DMF, stirred at room temperature for 72 hours, after the reaction was complete (thin-layer chromatography tracking), the reaction mixture was vigorously stirred and poured into 1000mL ice water, and continued to stir for 3 hours, a large amount of pyrimidine salicylaldehyde was separated out. Yield 95%.
[0060] The second step: add 13 grams (50mmol) of pyrimidine salicylaldehyde and 5.2 grams (50mmol) of sodium bisulfite in a 100mL three-necked flask with a thermometer, a reflux condenser, and a magnetic stirrer, and add 50mL of acetic acid / water mixed solution (1:4), heated to 40 degrees Celsius, kept for 0.5 hours, after the pyrimidine salicylaldehyde was completely dissolved, 6.85 grams (50 mmol) of p...
Embodiment 2
[0069] Synthesis of I-2:
[0070] The detailed experimental procedure is the same as in Example 1: add 4.06 grams (10 mmol) of 2-(4,6 dimethoxy)-pyrimidinyl oxide in a 100 mL three-necked flask with a thermometer, a reflux condenser, a magnetic stirrer and a dropping funnel Base-N-p-benzoyl-7-cyano-benzylamine, 2.82 grams (30mmol) of phenol, 0.122 grams (1mmol) of DMAP and 60mL of 1,2-dichloroethane, dropped into 10mL at 0 degrees Celsius to dissolve 2.26 grams of (11mmol) DCC solution in 1,2-dichloroethane was added in 30 minutes, and after the addition was completed, it was raised to room temperature and continued to stir for 24 hours. The reaction was complete (TLC tracking), filtered, and the filtrate was respectively washed with 1N HCl , saturated sodium bicarbonate, washed with saturated brine, dried over sodium sulfate, spin-dried, and separated by column chromatography to obtain a pure product with a yield of 85%.
[0071] solid
[0072] Melting point.: 127.5±0.5℃
...
Embodiment 3
[0078] Synthesis of I-3:
[0079] Add 4.06 grams (10 mmol) of 2-(4,6 dimethoxy)-pyrimidinyloxy-N-p-benzoic acid to a 100 mL three-necked flask with a thermometer, reflux condenser, magnetic stirrer and dropping funnel -7-cyano-benzylamine, 3.16 grams (30 mmol) of p-cresol, 0.122 grams (1 mmol) of DMAP and 60 mL of dichloromethane, stirred, and 10 mL of dichloromethane dissolved in 2.26 grams (11 mmol) of DCC was added dropwise at 0 degrees Celsius Methane solution was added in 30 minutes. After the addition was completed, it was raised to room temperature and continued to stir for 24 hours. The reaction was complete (TLC tracking), filtered, and the filtrate was washed with 1N HCl, saturated sodium bicarbonate, saturated brine, and sodium sulfate. Dried, spin-dried, and separated by column chromatography to obtain a pure product with a yield of 90%.
[0080] solid
[0081] Melting point: 81±1℃
[0082] 1 H NMR (300MHz, CDCl 3 / TMS): δ2.38(3H, s), 3.78(6H, s), 4.60(1H, d, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com