Targeted delivery of drugs for treatment of viral infections
A virus infection, antiviral drug technology, applied in the field of cells stressed by virus infection, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0030] According to the stoichiometric amount, the only one amino group of doxorubicin (DOX), that is, the reaction of one of the two reactive groups of the 3'-amino group and glutaraldehyde (GLU), is carried out with a predetermined molecular ratio, basically Large-scale synthesis of a homogeneous transferrin-doxorubicin conjugate. Therefore, the first step is to add DOX in saline solution dropwise to GLU in saline solution containing a solvent such as DMSO or other suitable cryoprotectants such that the final molar ratio of DOX and GLU is 1:1. The resulting DOX-GLU solution was stirred at room temperature in the dark for 3 hours.
[0031] In the above reaction, the molar numbers of DOX and GLU are equal, so that there is neither free DOX nor free GLU in the final obtained DOX-GLU solution. However, if one molecule of GLU and two molecules of DOX react to form DOX-GLU-DoX, there may be free GLU in the solution, but by adding monovalent DOX dropwise to the divalent GLU soluti...
Embodiment 1
[0050] Preparation of homogeneous transferrin-doxorubicin conjugates
[0051] According to the stoichiometric amount, utilize only one amino group of doxorubicin (DOX), that is, one of the two reactive groups of the 3′-amino group and glutaraldehyde (GLU), to react with a predetermined molecular ratio, basically Large-scale synthesis of homogeneous transferrin-doxorubicin conjugates. Therefore, the first step is to add DLU dropwise into DMSO while cooling with an ice-water bath. Next, the DOX in saline solution was added dropwise to the GLU+DMSO in saline solution so that the final molar ratio of DOX and GLU was 1:1. The resulting DOX-GLU solution was stirred at room temperature in the dark for 3 hours.
[0052] In the above reaction, the molar numbers of DOX and GLU are equal, so that there is neither free DOX nor free GLU in the final obtained DOX-GLU solution. However, if one molecule of GLU reacts with two molecules of DOX to form DOX-GLU-DOX, free GLU may exist in the ...
Embodiment 2
[0061] antiviral activity
[0062] The present invention was studied for its efficacy against different viruses. These viruses include cytomegalovirus (CMV), hepatitis B virus (HBV) and HIV. It turned out to be particularly effective against the HIV virus.
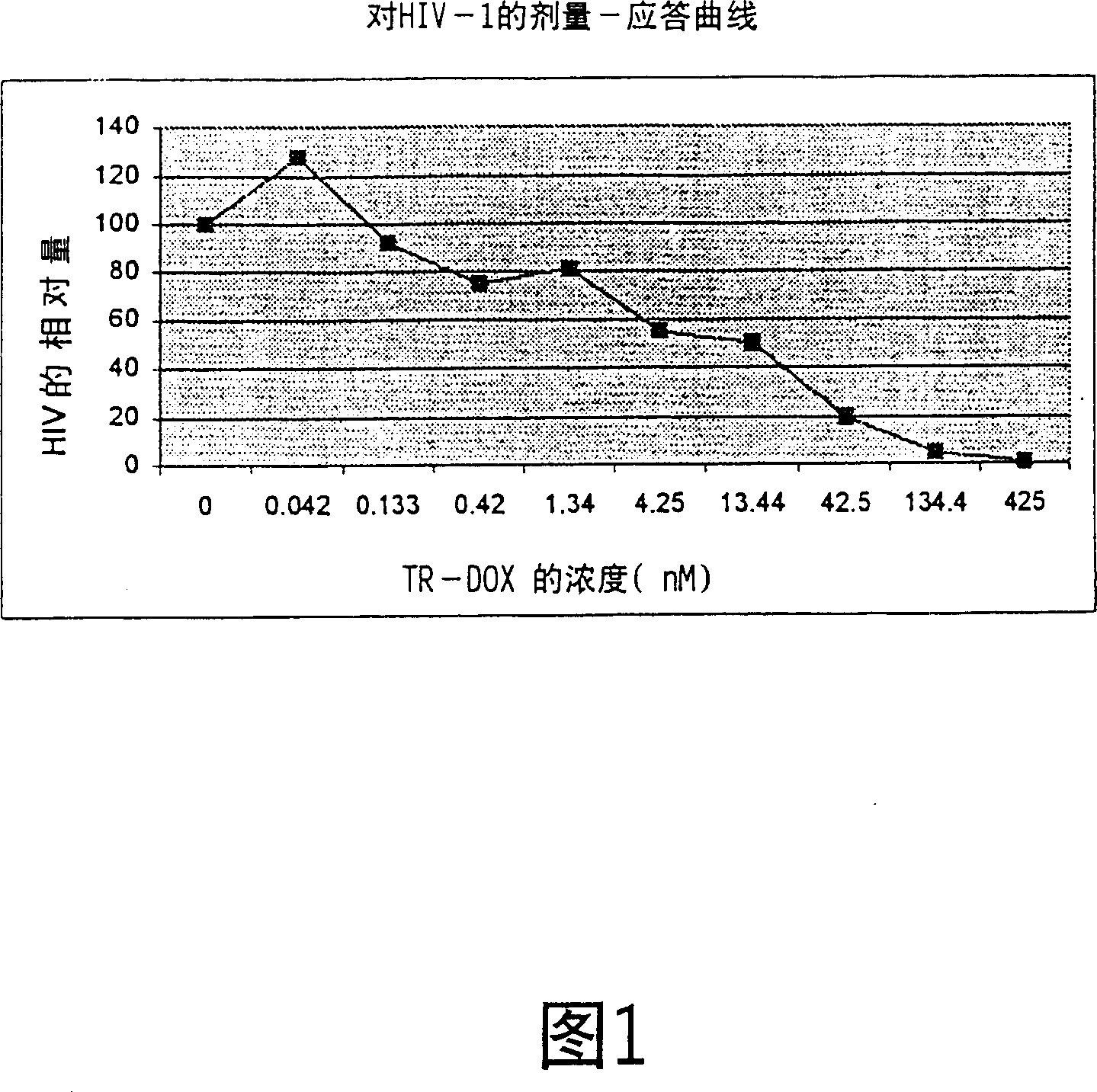
[0063] For example, as shown in Figure 1, a dose-response curve for the inhibition of ROJO strain of HIV-1 virus in human blood cells was obtained using TR-DOX conjugates. In the laboratory test system, the TR-DOX conjugate has a strong effect on the AIDS virus, and the concentration taken suggests that the TR-DOX conjugate can be used as an effective drug for treating HIV in AIDS patients.
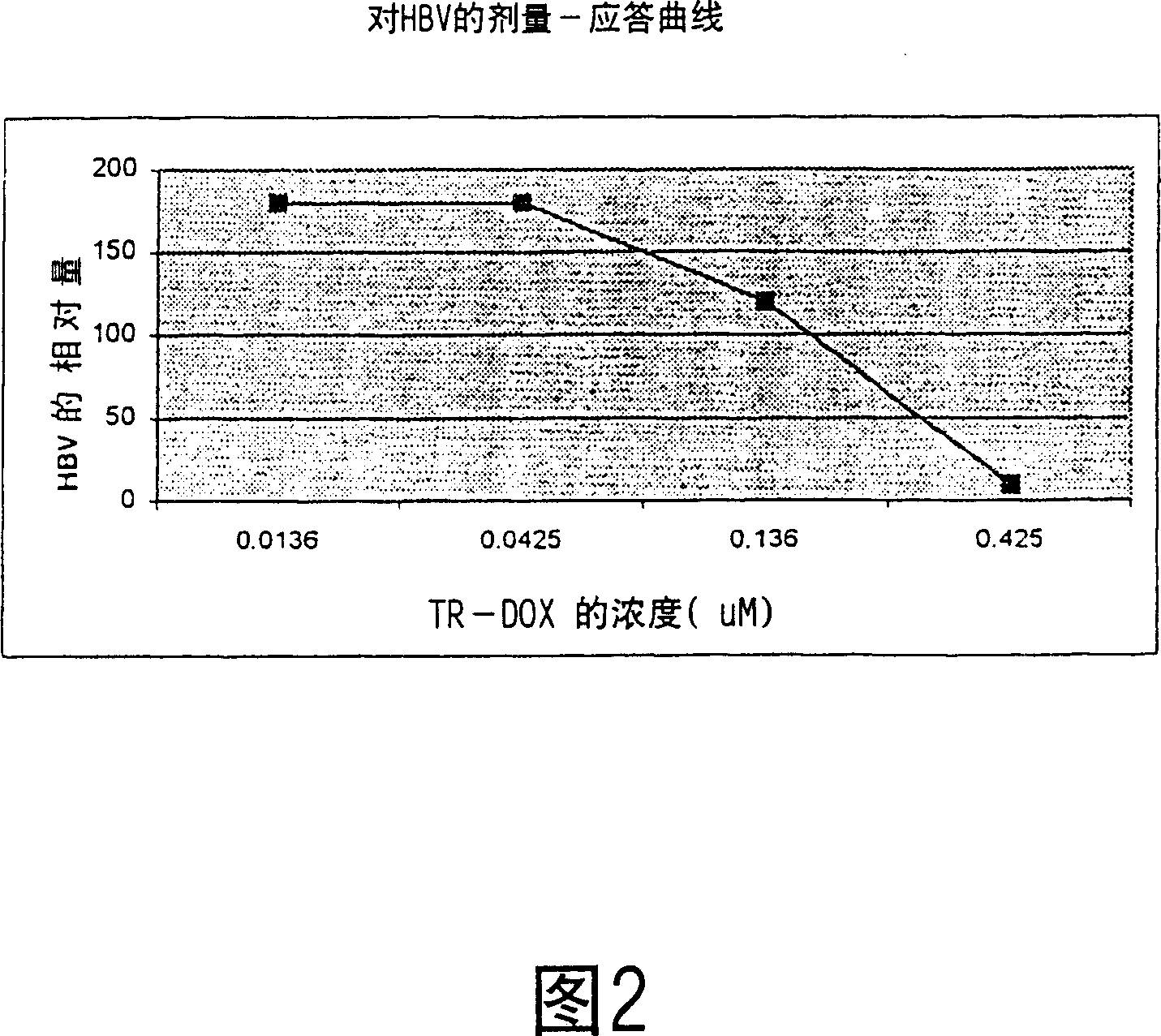
[0064] Similarly, Figure 2 shows dose-response curves of hepatitis B virus (HBV)-infected human liver cells exposed to increasing concentrations of transferrin-doxorubicin (TR-DOX) conjugates. Likewise, it has been found that very low concentrations of TR-DOX conjugates can substantially completely inhibit HBV.
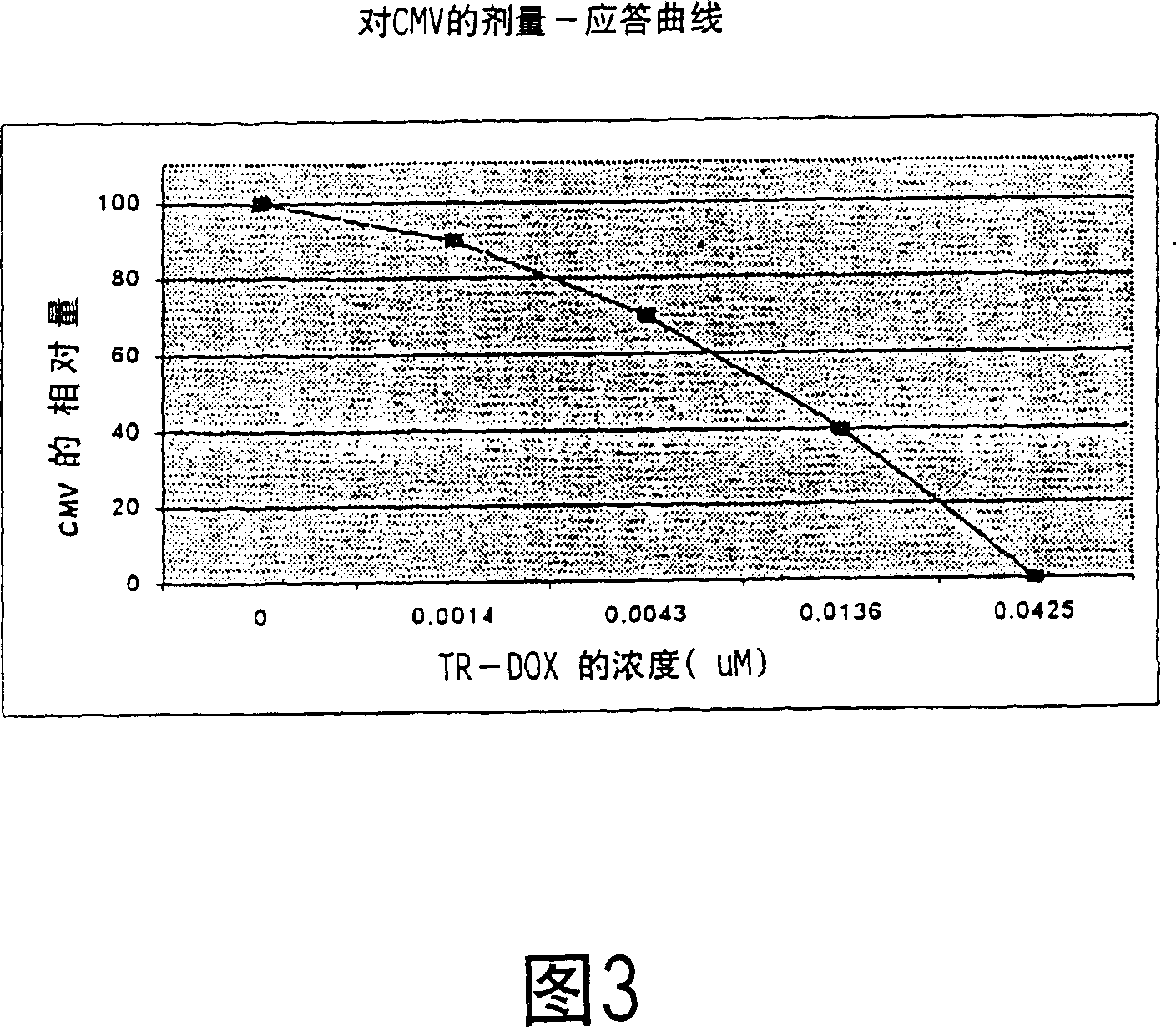
[0065] Finally, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com