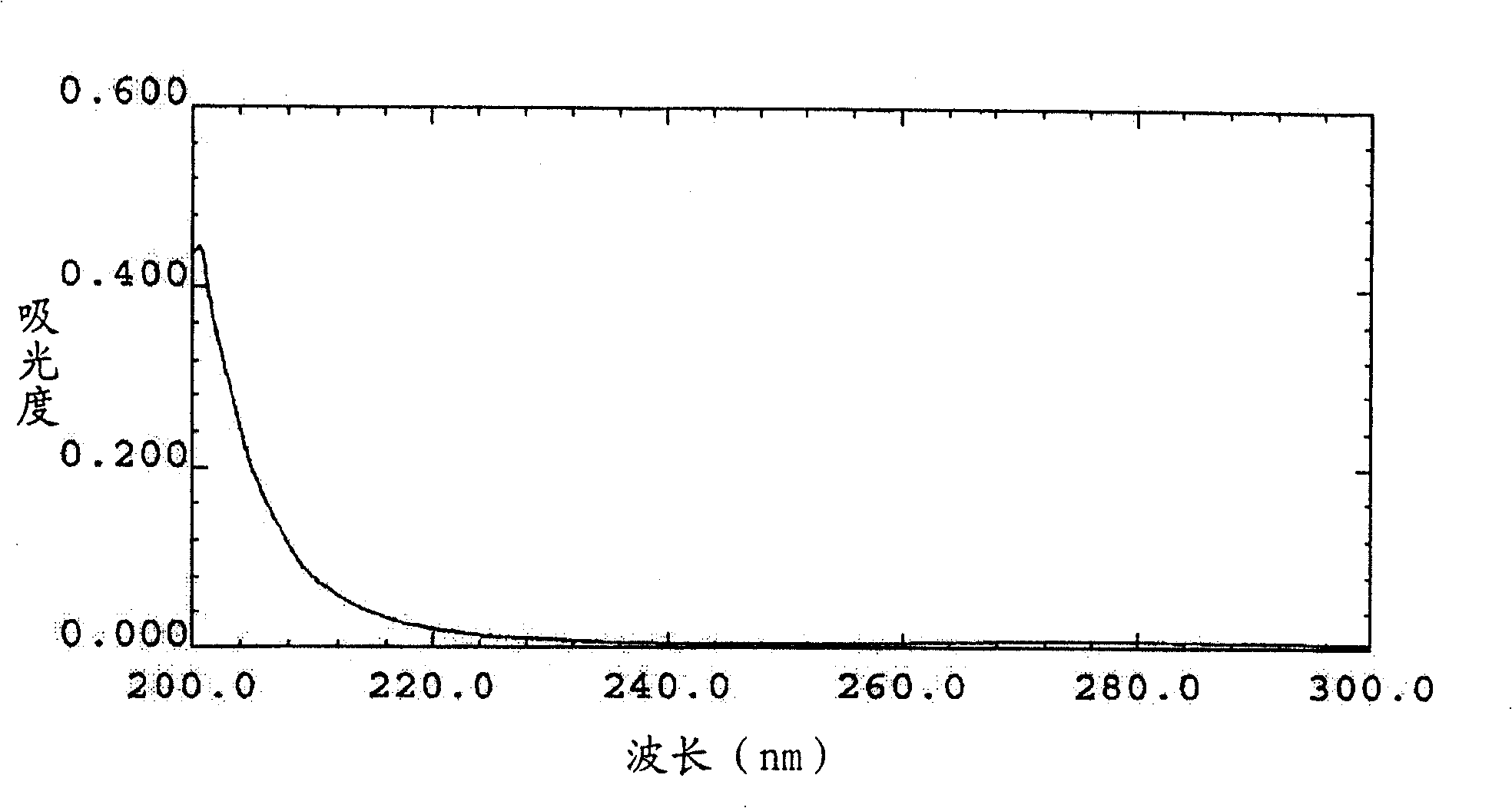
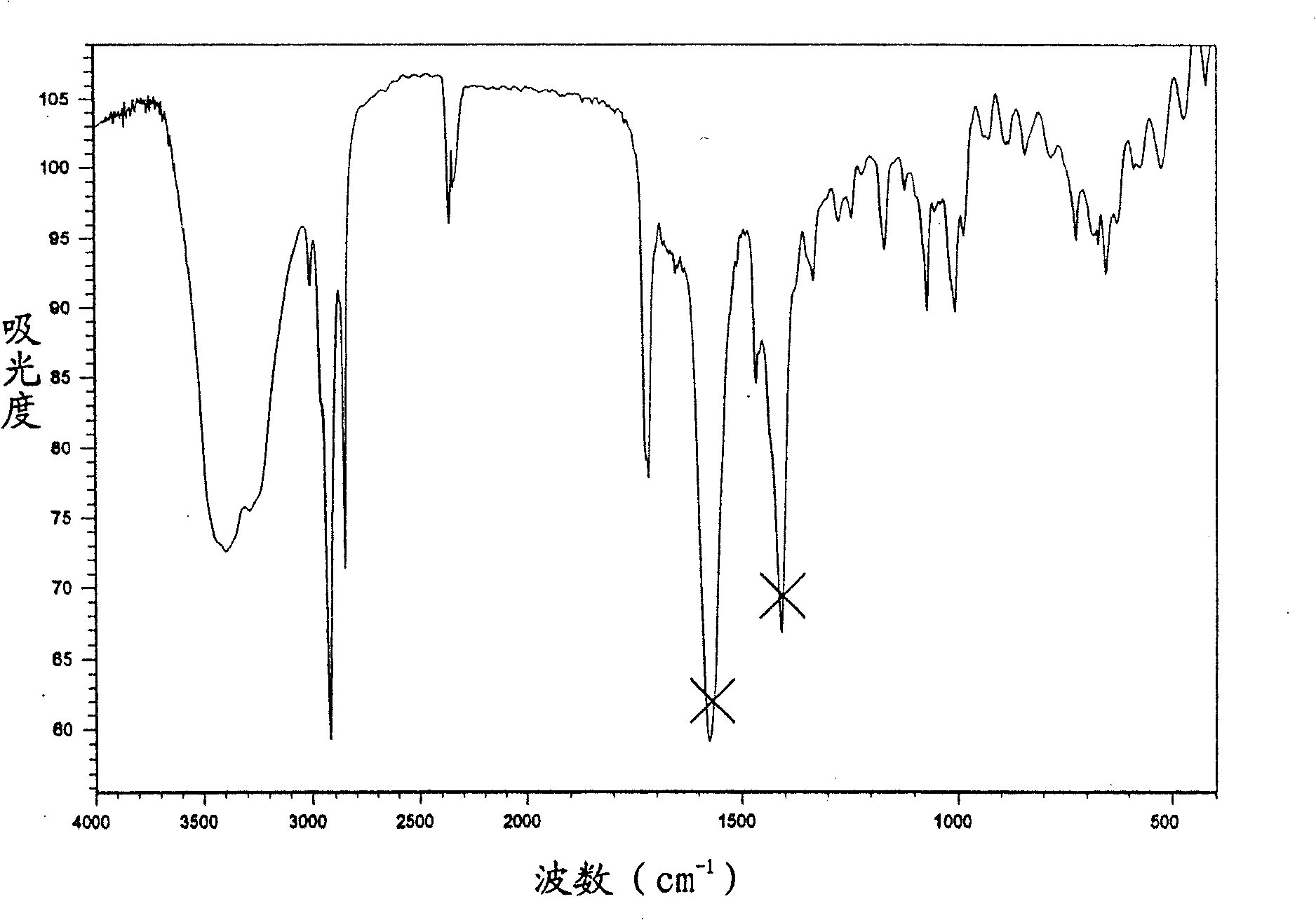
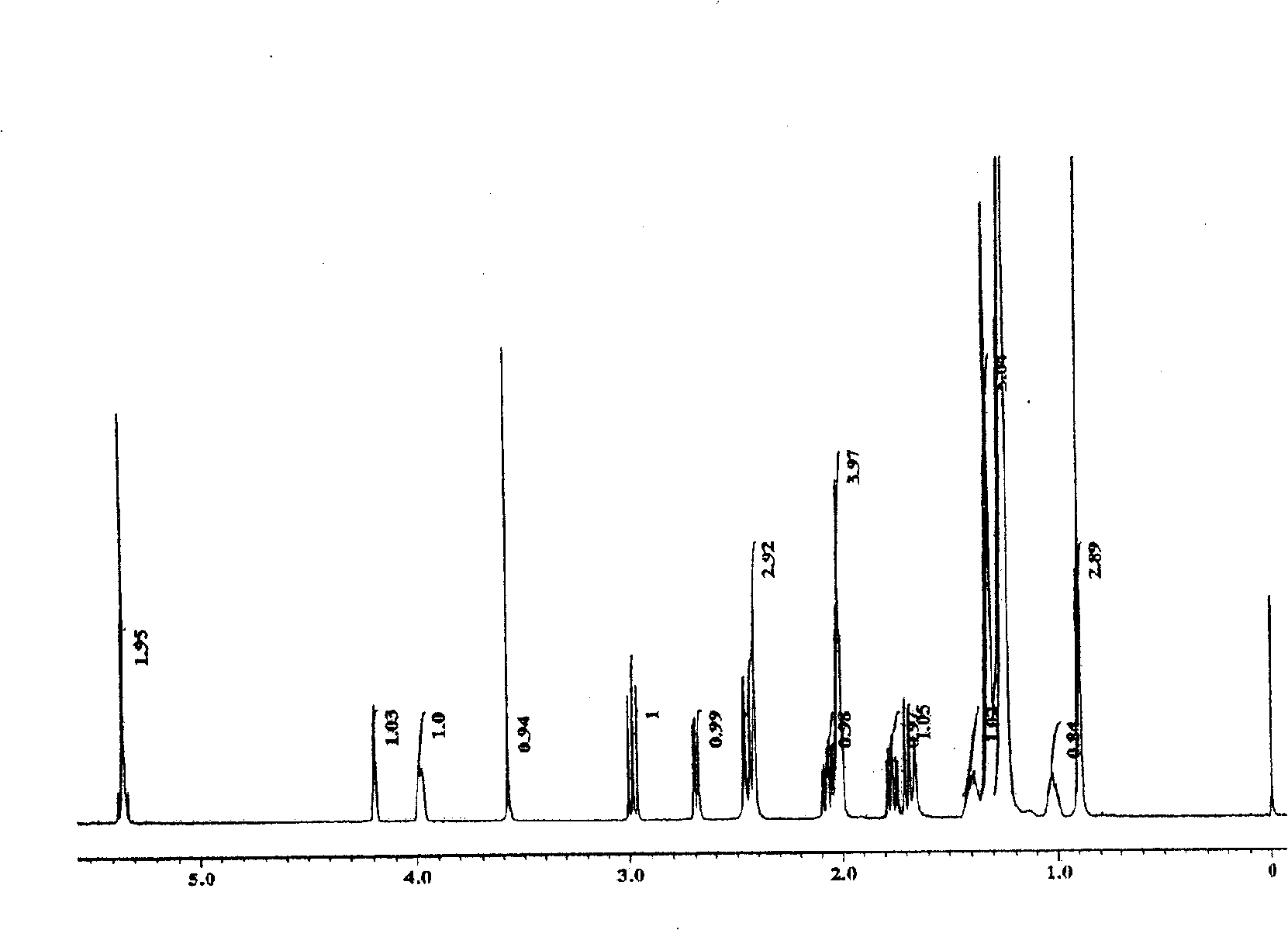
Compound of monocyclic polysubstitution saturated cyclohexanones, prepartion method and usage
A compound, cyclohexanone technology, applied in the direction of anhydride/acid/halide active ingredients, organic chemistry, drug combination, etc., can solve the problem of no research report on anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1. Extraction and separation and purification of compound I
[0071] The first step: preparation of the extract containing compound I
[0072] Take the dry bark (3.2kg) of Jujube jujube and grind it, and extract it with 25 liters of ethanol at room temperature, soaking for 7 days each time, and extracting 4 times in total. The extracts were combined, concentrated and dried to obtain 750 g of ethanol extract. Suspend 750 g of the ethanol extract in 3 liters of water, and extract 4 times with chloroform (3 liters), ethyl acetate (3 liters), and n-butanol (3 liters) in sequence to obtain 60 g of chloroform extract and 60 g of ethyl acetate extract, respectively. Extract 310g, n-butanol extract 300g and water layer residue 80g. Wherein, the chloroform extract is the extract containing compound I.
[0073] The second step: preparation of chromatographic crude fraction containing compound I
[0074] The chloroform extract (60 g) was dried on a silica gel column un...
Embodiment 2
[0082] Example 2. Extraction and separation and purification of compound II
[0083] The first step: preparation of the extract containing compound II
[0084] With reference to the first step in Example 1, the same method was used to obtain 110 grams of chloroform extract, the main extract containing compound II, from 6 kilograms of jujube raw materials.
[0085] Second step: preparation of chromatographic crude fraction containing compound II
[0086] Take 60 g of the chloroform extract containing compound II, put it on a vacuum silica gel column (column bed 7.5cm×18.5cm) by dry method, and elute with petroleum ether (P)-acetone (A) and methanol solvent system. The polarity of the elution solvent is gradually increased by increasing the amount of acetone (A) in petroleum ether (P) or replaced by methanol to increase the polarity of the elution solvent. Merge cuts according to TLC detection and activity test results, obtain 6 components Fr-1 (3.3g, V P :V A =100:1 elution...
Embodiment 3
[0094] Example 3. Biological activity test
[0095] Experimental samples and experimental methods
[0096] Preparation of the tested sample solution: respectively take the compound I separated and refined in the above-mentioned Example 1 and the compound II separated and refined in the embodiment 2, accurately weigh an appropriate amount, and prepare a solution of the required concentration with methanol for testing the activity.
[0097] Cell lines and cell culture: Mouse breast cancer tsFT210 cell line, human colorectal cancer HCT-15 cell line, human cervical cancer HeLa cell line, human breast cancer MCF-7 cell line, and human ovarian cancer A2780 cell line were used for activity testing.
[0098] Cells were subcultured in RPMI-1640 medium containing 10% FBS at 32°C (tsFT210 cells) or 37°C (HCT-15, HeLa, MCF-7 and A2780 cells) in an incubator filled with 5% carbon dioxide .
[0099] Cell proliferation inhibitory activity test method (Lissamine rhodamine B method or SRB me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com