Continuing release recipe of containing infusible basic remedy
A main drug, release-type technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as difficult mixing and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Granulation of controlled release tablets
[0020] All lozenge formulations were prepared by the general method of manufacture as described below. The clarithromycin, sodium alginate, lactose, and stearic acid were all passed through a No. 40 sieve to remove any large lumps, and the sieved material was mixed in a Supermixer for 20 minutes, then slowly A solution of povidone and citric acid was added to form suitable granules. The wet granules passed through a No. 16 sieve, and were dried with hot air at 60°C until the moisture content of the granules was 5-7% as measured by a Karl Fisher moisture analyzer. After passing through a No. 20 screen, the dried granules were mixed with lubricant on a V-blender for 2 minutes.
[0021] 2. Ingot making
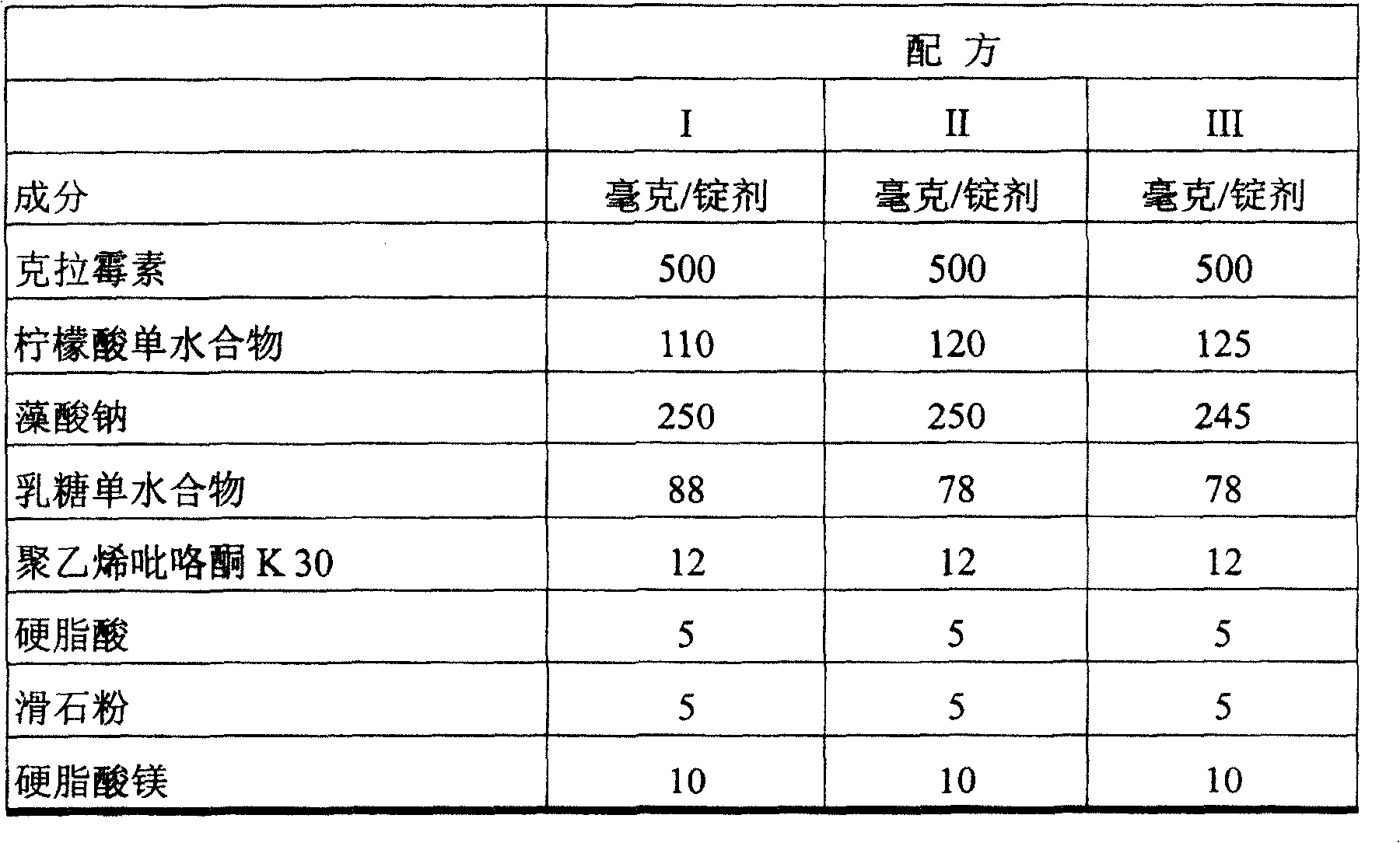
[0022] The rotary tablet machine was equipped with an oval mold, and the tablet formulations I, II and III shown in Table 1 below were respectively pressed into suitable thickness and crispness. The composition of the loze...
Embodiment 2
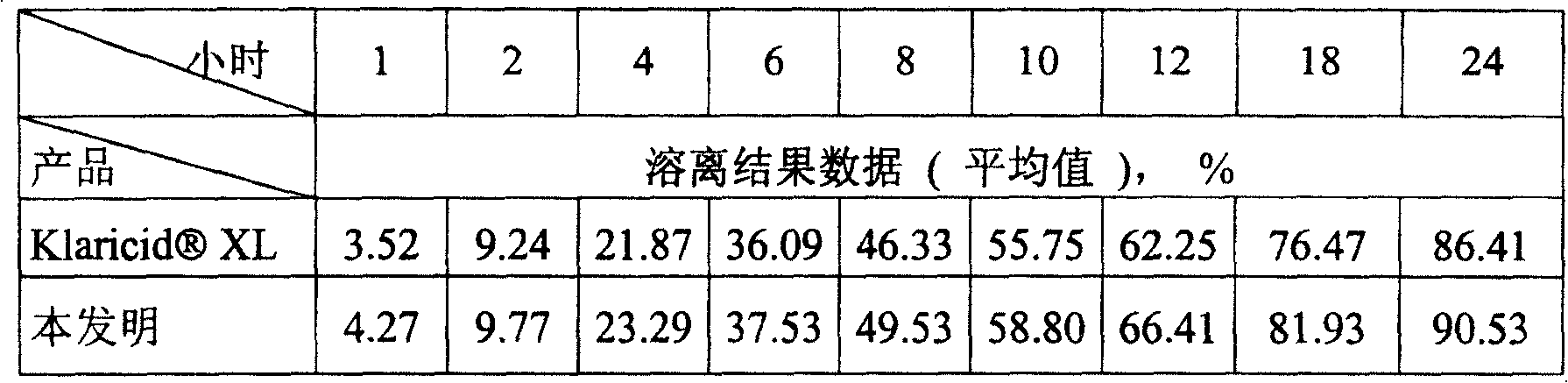
[0028] Solubility studies
[0029] 1. Solubility test, according to page 463 of the 27th edition of the United States Pharmacopoeia (2004)
[0030]0.1M sodium acetate buffer solution: the preparation method is as follows: take 13.61g of sodium acetate trihydrate, put it in a 1-liter volumetric flask, add water to dissolve it and dilute to volume, mix well, and adjust its pH value to 5.0 with 0.1M acetic acid. Solvent: 900 mL of the above 0.1 M sodium acetate buffer solution.
[0031] Device 2: 50rpm
[0032] Sampling time points: 1st, 2nd, 4th, 6th, 8th, 10th, 12th, 18th and 24th hours.
[0033] Mobile phase: Its preparation method is: take a mixture of methanol and 0.067M potassium dihydrogen phosphate solution (the ratio of methanol to 0.067M potassium dihydrogen phosphate solution is 650:350), add phosphoric acid to adjust its pH to 4.0, and use a pore size of 0.5 μm or a finer filter and degassed, and adjusted if necessary.
[0034] Standard solution: The preparation m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com