Recombinant Plasmodium falciparum 175kD erythrocyte conjugated antigen functional region protein and its preparation and use
A recombinant protein and antigen-binding technology, which is applied in the field of DNA recombination technology and genetic engineering vaccines, can solve problems such as the advent of vaccines, the failure to express 175kD red blood cell-binding antigen, and the inability to implement and apply anti-malarial immunogens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
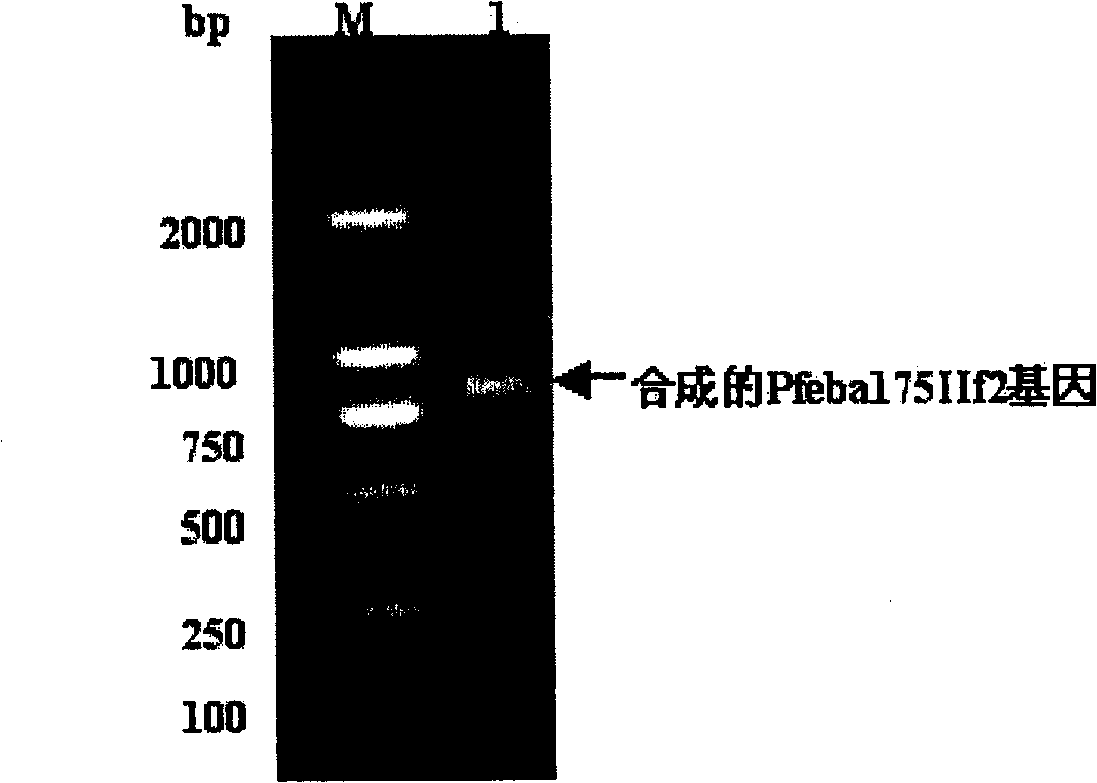
[0078] Synthesis of PfEBA-175IIF2 Gene
[0079] 1.1. Design of Pfeba-175IIf2 Totally Synthetic Gene
[0080] The sequence of the recombinant EBA-175IIF2 protein is derived from the amino acid sequence of the 175kD erythrocyte-binding antigen functional region EBA-175IIF2 of the Plasmodium falciparum 3D7 strain. The sequence was redesigned by selecting the codon usage frequency of Pichia pastoris. Use computer software "DNA Star", Omiga, etc. to analyze and exclude any sequences in the gene sequence that may be unfavorable to gene transcription and translation, such as transcription termination sequences, intron splice site sequences, long inverted repeat sequences Wait. In addition, XhoI and EcoRI restriction sites were added at the 5' and 3' ends of the newly designed eba-175IIf2 gene, respectively, so as to be connected with the expression vector. A potential glycosylation site in the antigen sequence was identified and excluded by converting the glycosylation site amino ...
Embodiment 2
[0097] Secretion and expression of Pfeba-175IIf2 gene in Pichia pastoris
[0098] 2.1: Construction of expression vector
[0099] Pfeba-175IIf2 gene was inserted into pPIC9 yeast expression plasmid (purchased from Invitrogen Company) through XhoI and EcoRI sites. In this way, the N-terminal of PfEBA-175IIF2 is fused with the C-terminal of the signal peptide of Saccharomyces cerevisiae α-factor on the vector. Because the N-terminal of the PfEBA-175IIF2 molecule contains three specific amino acid Glu-lys-Arg sequences of the signal peptide cutting point. Therefore, the target protein released by secretory expression does not contain a signal peptide sequence.
[0100] The basic elements of the pPIC9K vector are the same as pPIC9, but it has a kanamycin resistance gene, and G418 can be used for screening of high-copy inserts. In this way, the fragment containing Pfeba-175IIf2 was cut out with BamHI and SalI and inserted into the corresponding site of the pPIC9K expression plas...
Embodiment 3
[0106] Fermentation and purification of PfEBA-175IIF2
[0107] After optimizing the expression conditions, the expression level of shake flask can reach 20mg / L. Fermentation expression was performed in 15L tanks. During the whole fermentation cycle, the cells in the early stage grow exponentially, and the cell density reaches OD 600 =100. In the growth phase of glycerol addition, the cell growth increased linearly, and the cell density reached OD 600 =560. However, in the stage of methanol-induced expression, the total cell density remains basically unchanged, while the expression of the target protein begins 3-7 hours after the addition of methanol, and as time goes on, the expression yield increases rapidly and is continuously secreted into the fermentation broth. The amount can reach 300mg / L.
[0108] The purification of PfEBA-175IIF2 expression product was carried out in two steps: the first step was purification with Ni-NTA column. Since the C-terminus of PfEBA-175I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com