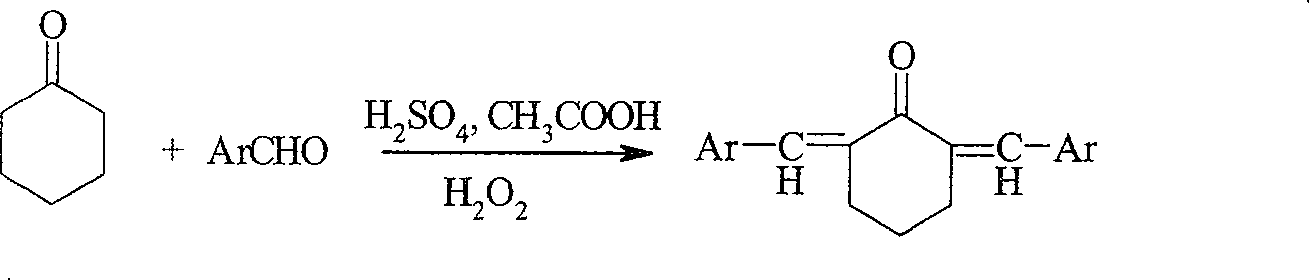Method of preparing alpha, alpha'-di(substituted benzylidene)cyclohexanone ultraviolet radiation absorbent
An ultraviolet and absorber technology, applied in the field of ultraviolet absorbers, can solve the problem that the sunscreen effect is not very high, and achieve the effect of stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
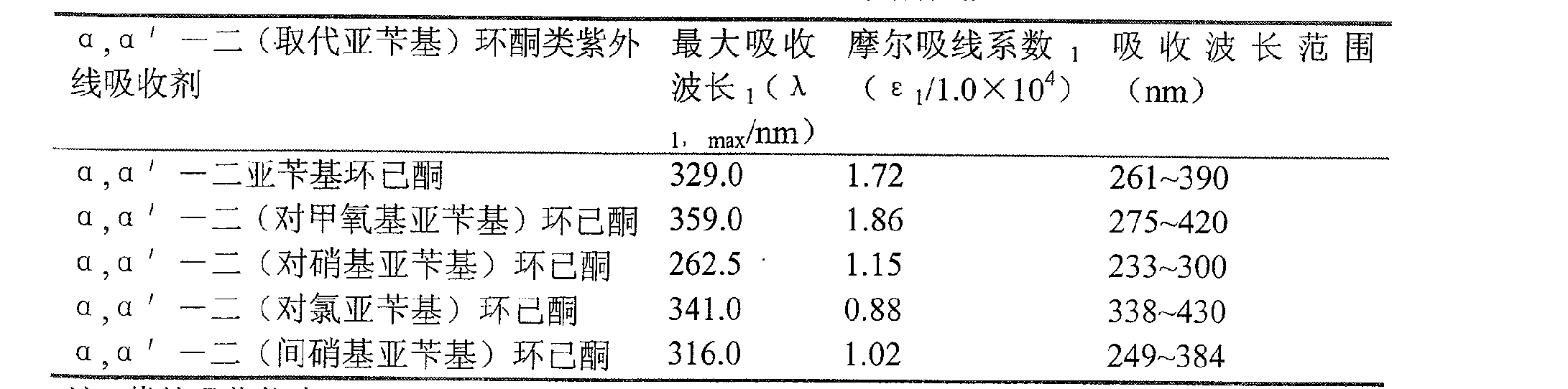
[0024] Add 0.5ml of concentrated sulfuric acid, 7ml of acetic acid and 0.25ml of 30% hydrogen peroxide into a 100ml three-necked bottle (the volume ratio of concentrated sulfuric acid: acetic acid: 30% hydrogen peroxide is 2:28:1), when concentrated sulfuric acid, acetic acid, 30% hydrogen peroxide mixture After cooling to room temperature (20-25°C), slowly add the mixture of cyclohexanone and benzaldehyde, the molar ratio of ketone and aromatic aldehyde in the mixture is 1:2, after stirring at room temperature for about 7 hours, there is a large amount of Precipitation was formed, after adding 40-60ml of water, the solid was filtered out with suction, washed with distilled water, and then rinsed with aqueous methanol (the volume ratio of methanol:water was 1:1). The obtained solid was recrystallized from dichloromethane and ethanol respectively. The corresponding product α,α'-dibenzylidene cyclohexanone was obtained. The solid was weighed after drying, and the yield calculat...
Embodiment 2
[0026] Add 4ml concentrated sulfuric acid, 20ml acetic acid and 2ml hydrogen peroxide (the volume ratio of concentrated sulfuric acid: acetic acid: 30% hydrogen peroxide is 4:20:2) in a 100ml three-necked bottle, control the reaction temperature to 35°C, slowly add cyclohexanone dropwise Mixed solution with m-nitrobenzaldehyde, the molar ratio of ketone and m-nitrobenzaldehyde in the mixed solution is 1:2, after stirring at 35°C for about 20 hours, add 50% sodium hydroxide solution to neutralize the reaction solution to medium After allowing the reaction solution to stand for 3 to 4 hours, a large amount of precipitate formed, and the solid was filtered out with suction and washed with 5 to 10 ml of ethyl acetate. The resulting solid was recrystallized from dichloromethane and ethanol, respectively. The corresponding product α,α'-bis(m-nitrobenzylidene)cyclohexanone is obtained. The solid was weighed after drying, and the yield calculation and structure identification were ca...
Embodiment 3
[0028] Add 2ml of concentrated sulfuric acid, 14ml of acetic acid and 1ml of hydrogen peroxide into a 100ml three-necked bottle (the volume ratio of concentrated sulfuric acid: acetic acid: 30% hydrogen peroxide is 2:14:1), control the reaction temperature to 35°C, and slowly add cyclohexanone dropwise Mixed solution with p-chlorobenzaldehyde, the molar ratio of ketone and aromatic aldehyde in the mixed solution is 1:1.6, after stirring at 45°C for about 10 hours, add 50% sodium hydroxide solution to neutralize the reaction solution to neutral, let the reaction After the solution was left to stand for 3 to 4 hours, a large amount of precipitate formed, and the solid was filtered out with suction and washed with 5 to 10 ml of ethyl acetate. The resulting solid was recrystallized from dichloromethane and ethanol, respectively. The corresponding product α,α'-bis(p-chlorobenzylidene)cyclohexanone is obtained. The solid was weighed after drying, and the yield calculation and structu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com