Gynecopathy treating formulation and preparation process thereof
A technology for pharmaceutical preparations and gynecological diseases, which is applied in the field of pharmaceutical preparations for the treatment of gynecological diseases and its preparation. It can solve the problems of large doses of granules, low bioavailability, and backward dosage forms, and achieve improved bioavailability and bioavailability. High, increased stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0019] Experimental Example 1: Research on Extraction Process
[0020] 1. Selection of decoction and extraction conditions of medicinal materials
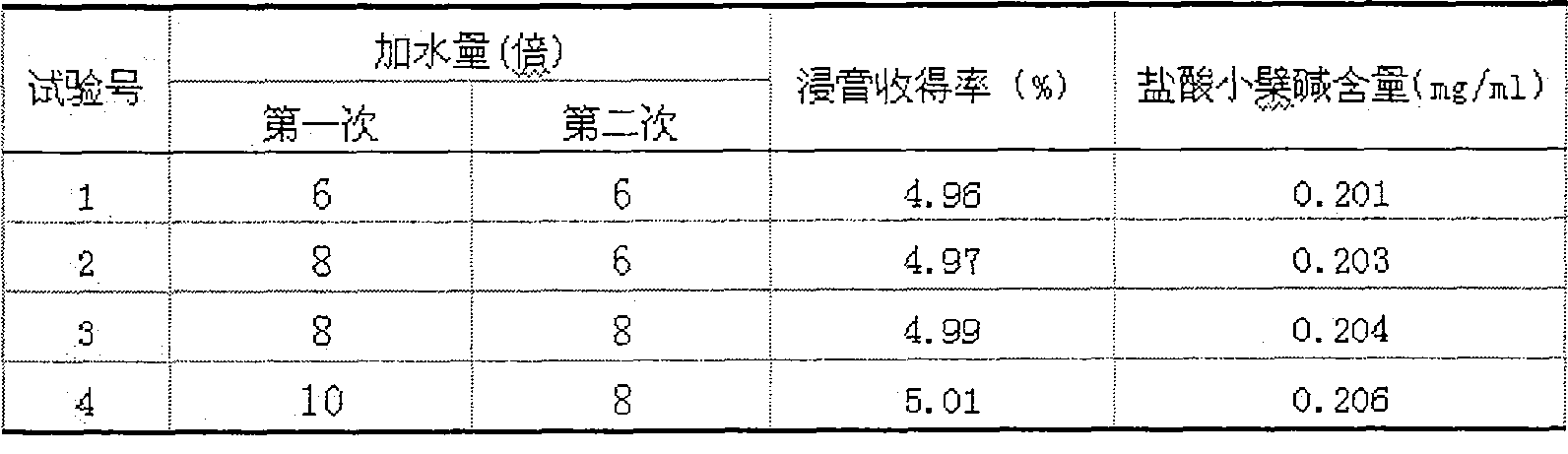
[0021] (1) Factor selection The decoction and extraction effect of traditional Chinese medicine is affected by factors such as the amount of water added, extraction time, and extraction times. The applicant selects the amount of water added as a factor, and focuses on the influence of different levels of factors on the decoction extraction effect. Combined with production cost, energy and other aspects to comprehensively consider, select the factor level.
[0022] (2) Index Determination Select two items of extract yield and berberine hydrochloride content as evaluation index, its reason and measuring method are as follows:
[0023] ① Yield rate of extract: extract is the material basis for solid preparations to exert their curative effect, and its yield directly affects the preparation process, so choosing it as an extraction in...
experiment example 2
[0036] Experimental Example 2: Study on Separation, Concentration and Drying Process
[0037] Separation process research: In order to facilitate production operation control, the extract was filtered with a 200-mesh filter cloth, and the extract was combined.
[0038] Concentration process research: use three-effect concentration tanks for concentration, and the extract is concentrated to a thick paste with a relative density of 1.30 (60°C). The degree is -0.025Mpa for the first effect, -0.045Mpa for the second effect, and -0.065Mpa for the third effect.
[0039] Drying process research: vacuum drying conditions: 60 ~ 70 ℃, -0.08Mpa.
[0040] The results show that the extraction process of the present invention is reasonable and feasible.
experiment example 3
[0041] Experimental Example 3: Research on Molding Process
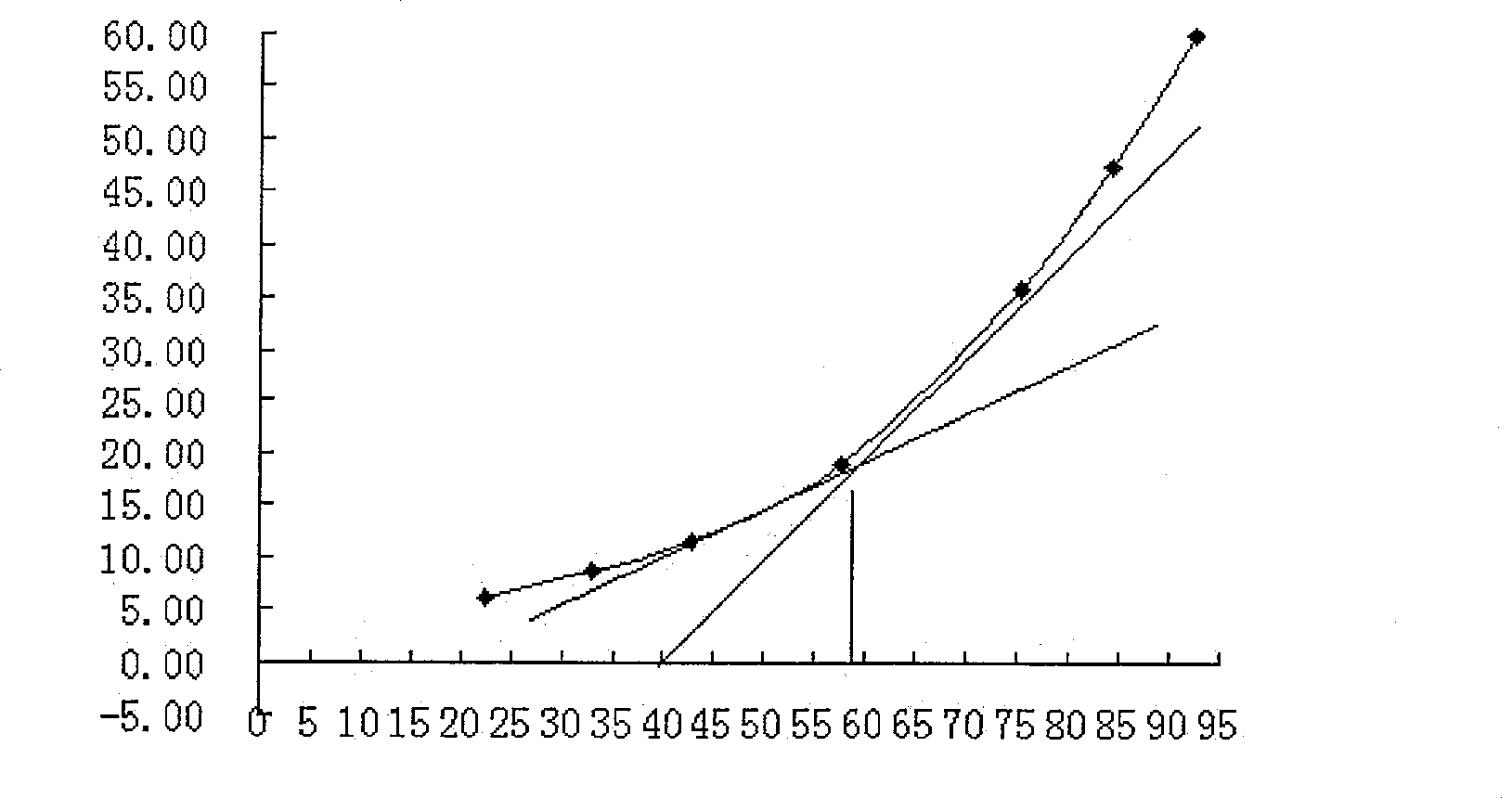
[0042] Angle of repose measurement: Use the fixed funnel method, fix the funnel on the coordinate paper placed horizontally, the distance between the lower opening of the funnel and the coordinate paper is 3cm, pour different prescription pills into the funnel, until the tip of the cone formed at the bottom touches Measure the diameter of the bottom of the cone to the mouth of the funnel below, and calculate the angle of repose. A small angle of repose indicates good fluidity of the particles.
[0043] Disintegration time limit inspection: use the rotating basket method, lifting disintegration instrument, take 6 tablets / capsules of tablets or capsules, and observe the situation of passing through the sieve. The higher the pass rate, the better the disintegration, which is more suitable for human body absorption.
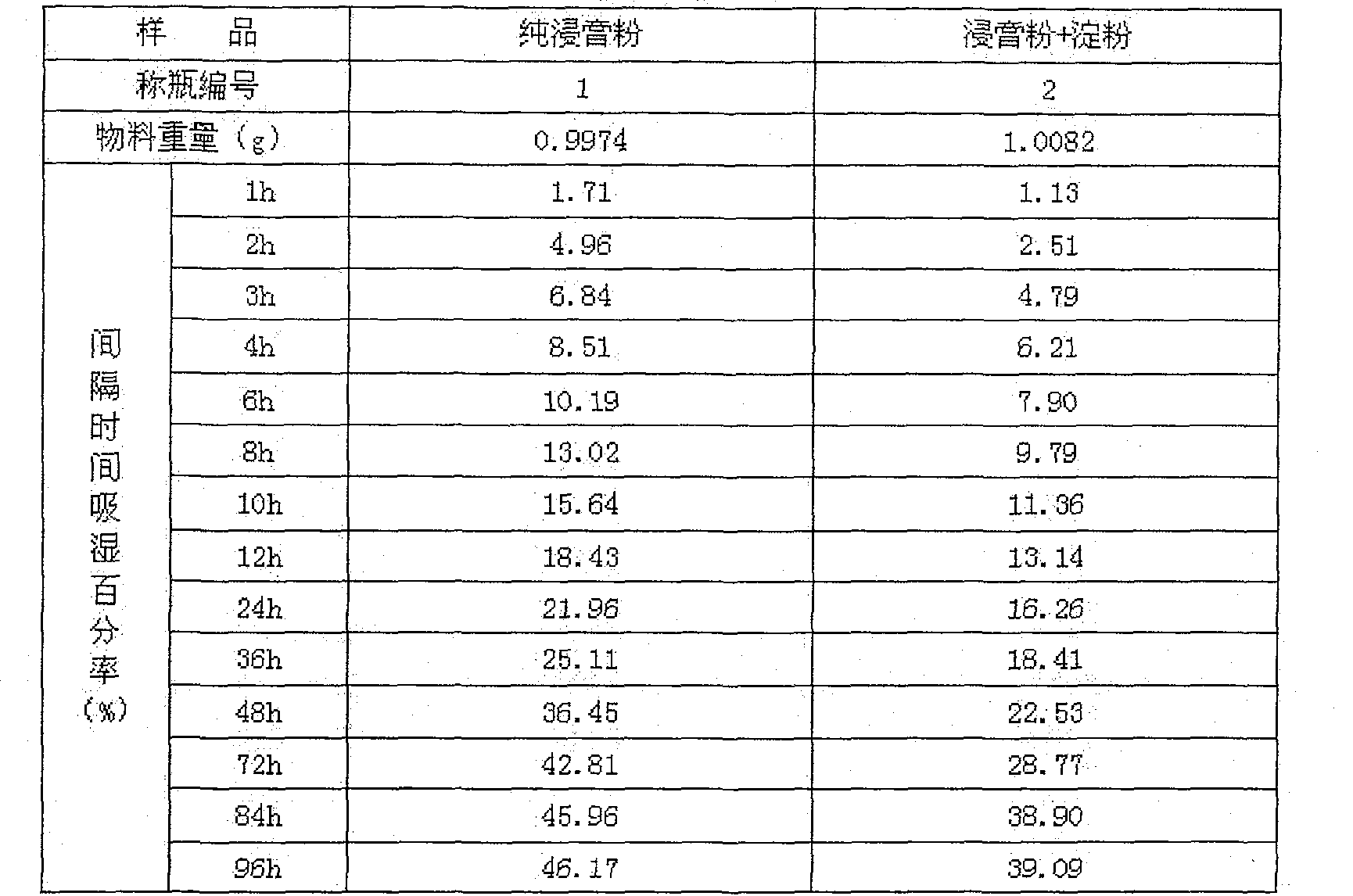
[0044] Solubility test: Take 10g of granules, heat water 20 times, stir for 5 minutes, and observe imme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com