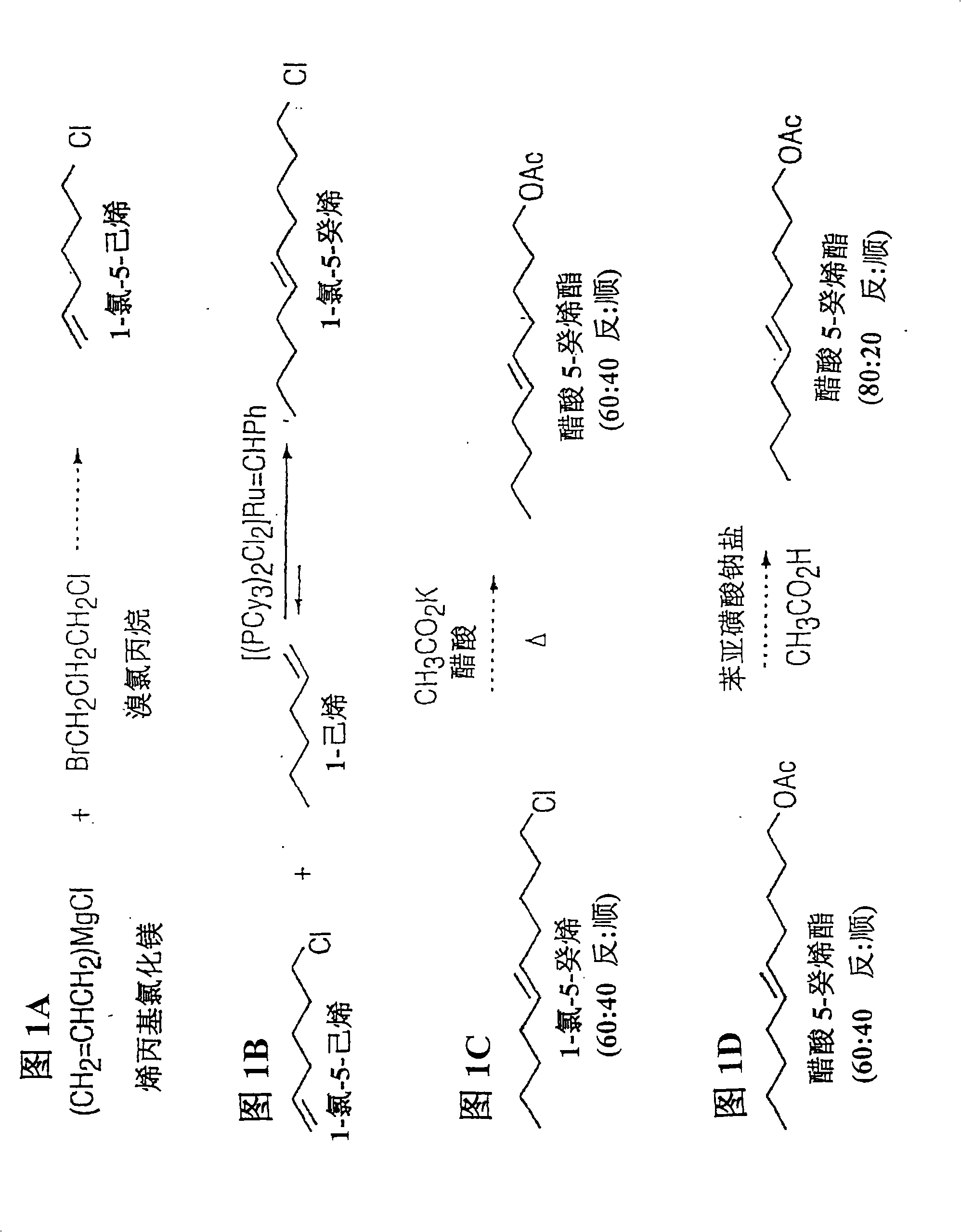
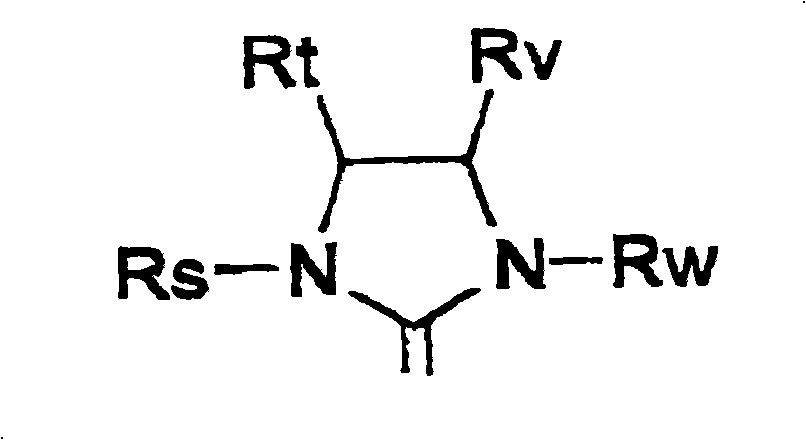
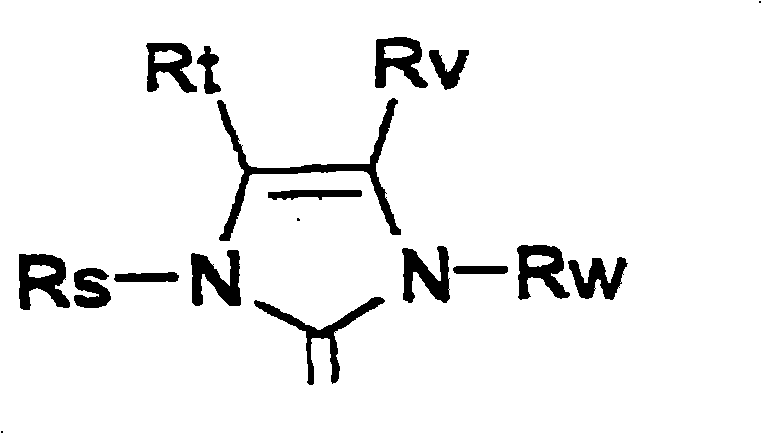
Metathesis synthesis of pheromones or their components
A metathesis and cross-metathesis technology, which is applied in the direction of metathesis reactions to produce hydrocarbons, compounds containing elements of group 5/15 of the periodic table, compounds containing elements of group 3/13 of the periodic table, etc., can solve the problem of expensive and easy-to-produce drug resistance And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Synthesis of 5-decene: Self-metathesis of 1-hexene
[0126] refer to Figure 9A , to a dry 2-L round bottom flask was charged 225 g (2.67 mol) of 1-hexene (obtained from Amoco with a purity greater than 95%) and a magnetic stir bar. The flask was sparged with nitrogen for 10 minutes. Catalyst 823 (2.2 g, 2.7 mmol) was added, and the reaction was stirred at room temperature for 18 hours. Ethylene gas was observed to evolve from the reaction. Spent catalyst was removed by filtering the reaction through 200 g of 60-200 mesh J.T. Baker Silica Gel in a 1.5 inch x 22 inch chromatography column. The chromatographic column was washed with 300 mL of petroleum ether (38°C to 55°C boiling point). The solvent and unreacted 1-hexene were removed under reduced pressure to yield 115 g (0.81 mol) of 5-decene. This product was used in the next reaction without further purification.
[0127] 5-Decenyl Acetate Synthesis: Cross-Metathesis 5-Decene and 5-Hexenyl Acetate
[0128] ...
Embodiment 2
[0145] Synthesis of 5-Decene: 1-Hexene Self-Metathesis
[0146] refer to Figure 9A , to a clean 72 L round bottom flask (high efficiency reflux condenser with -10 °C circulating coolant) attached to a pneumatic overhead stirrer was added 48 L (384 mol) of 1-hexene (obtained from Amoco, purity over 99 percent, using without further purification). Stirring was started and the solution was sparged with nitrogen from subsurface for 15 minutes. Catalyst 823 (10 g, 0.018 mol) was added and stirred under nitrogen for 18-24 hours. Ethylene is discharged into the exhaust pipe through the high-efficiency condenser.
[0147] After 24 hours, GC analysis indicated 60 to 70 conversion of 1-hexene to 5-decene. The reaction mixture was passed through 2.5Kg of silica gel (Fisher 170-400 mesh, ) filter to remove the catalyst used.
[0148] The skilled person will appreciate that the starting material can be used in the next reaction without purification of the intermediate compound. ...
Embodiment 3
[0170] Synthesis of 5-Decenyl Acetate Using Catalyst 848
[0171] Also refer to Figure 9A , produce 5-decene as above embodiment 1 or 2, perhaps with catalyst 848 ( Figure 5A ) instead of catalyst 823.
[0172] Also refer to Figure 9B , to a 100 mL round bottom flask with a magnetic stir bar and vacuum adapter was added 10 g (70.4 mmol) 5-hexenyl acetate and 30 g (214 mmol) 5-decene. The reaction was sparged with nitrogen for 5 minutes, then 20 mg of Catalyst 848 was added and stirred under 10 mm Hg vacuum for 45 minutes.
[0173] The metathesis catalyst was removed as previously described, yielding a clear liquid. GC analysis indicated 78% conversion of 5-hexenyl acetate to 5-decenyl acetate with an E:Z isomer ratio of 82:18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com