Pharmaceutical preparation of povidone iodine and preparation method thereof
A technology of povidone-iodine and pharmaceutical preparations, which is applied in the directions of botanical equipment and methods, pharmaceutical combinations, pharmaceutical formulations, etc., can solve the problems of inability to maintain the drug concentration for a long time, poor stability of the solution, easy volatility and the like, and avoid hypertonicity. Dehydration, suitable osmotic pressure, highly volatile effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~2
[0034] The molecular weight of the polyethylene glycol of embodiment 1 is 4000, and the molecular weight of the polyethylene glycol of embodiment 2 is 6000
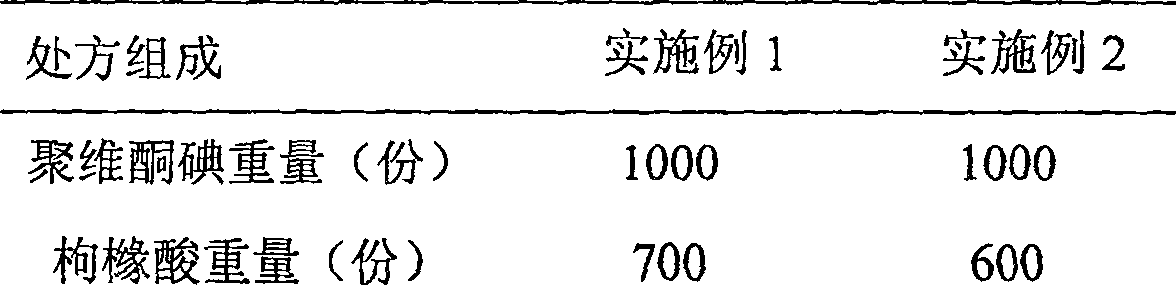
[0035] Weigh povidone iodine, grind povidone iodine and citric acid, stir and mix, then grind, stir and mix with sodium bicarbonate, finally add polyethylene glycol, mix, sieve, and tablet . Each component in the tablet is shown in Table 1 below, and the consumption is weight (parts).
[0036] Table 1
[0037]
[0038]
[0039] Weigh 271 parts by weight of phosphoric acid, 236 parts by weight of acetic acid, 247 parts by weight of boric acid, 280 parts by weight of sodium hydroxide, and 90-120 parts of methylparaben and mix them, then add 99000 parts by weight of deionized water, and adjust the pH with sodium hydroxide The value was 5.8 ± 0.2, obtaining a liquid phase buffer.
[0040] Put the tablets obtained in Examples 1-2 together with the liquid-phase buffer solution in a kit and pack them in isolation. When ...
Embodiment 3
[0043] Weigh 271 parts by weight of phosphoric acid, 236 parts by weight of acetic acid, 247 parts by weight of boric acid, 280 parts by weight of sodium hydroxide, and 100 parts of methylparaben and mix them, then add 99000 parts by weight of deionized water, and adjust the pH value with sodium hydroxide. 5.8 ± 0.2, to obtain the liquid phase buffer.
[0044] Put the tablets obtained in Examples 1-2 together with the liquid-phase buffer solution in a kit and pack them in isolation. When in use, take out the solid tablets and put them in the buffer solution. After they are completely dissolved, they can be used directly.
[0045] The weight ratio of the tablet to the liquid phase buffer is: solid tablet part: liquid phase buffer part = 1:40.
Embodiment 4
[0047] Stability test:
[0048] Carry out stability test respectively to prepared povidone-iodine tablet, test method:
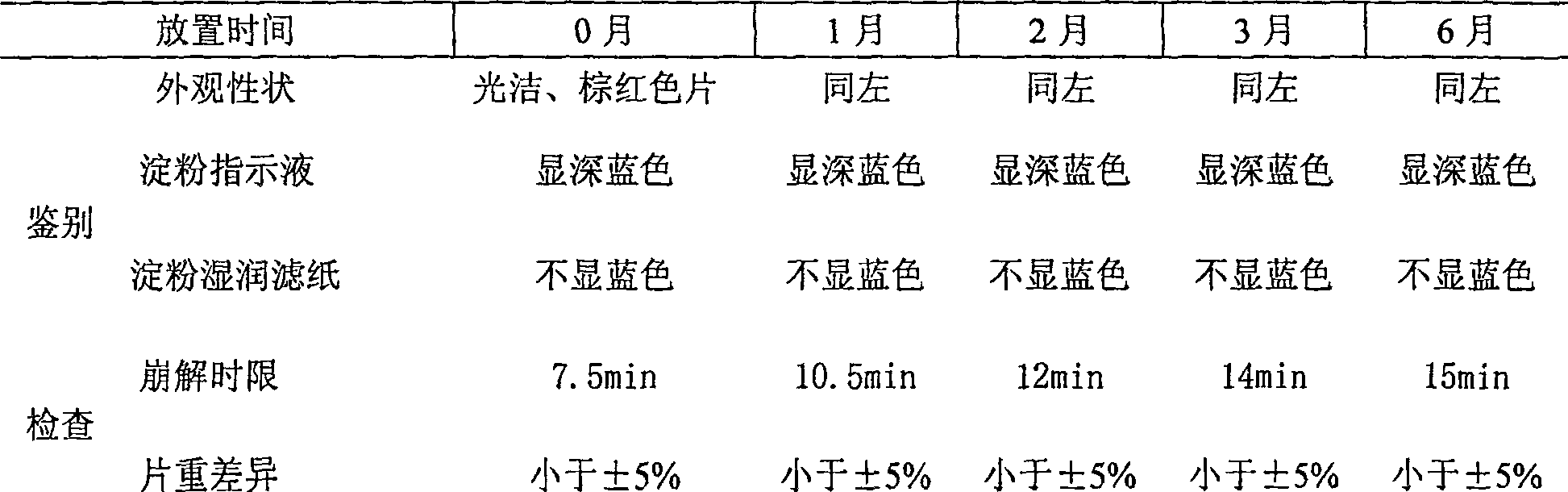
[0049] Place the tablet in a sealed commercially available package, put it in a constant temperature oven at a temperature of 40±2°C, conduct an accelerated test, and take samples for measurement at 0, 1, 2, 3, and 6 months. The test items include: Appearance, identification, inspection (difference in disintegration time and tablet weight), content determination. This tablet is investigated 6 months by the constant temperature accelerated test method, and the results are as follows:
[0050] Table 2 Accelerated test report form
[0051] Sample name: Povidone-iodine tablets Lot number: 060619
[0052]
[0053]
[0054] Table 3 Accelerated test report form
[0055] Sample name: Povidone-iodine tablets Lot number: 060620
[0056]
[0057] Table 4 Accelerated test report form
[0058] Sample name: Povidone-iodine tablets Lot ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com