Method for preparing nano-scale lithium carbonate for battery
A nano-scale, lithium carbonate technology, applied in the direction of lithium carbonate;/acid carbonate, etc., can solve the problem that lithium carbonate cannot be used in battery electrolyte, etc., and achieve the effect of improving battery capacity and low-temperature discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] This example is used to illustrate the preparation method of nanoscale lithium carbonate for batteries provided by the present invention.
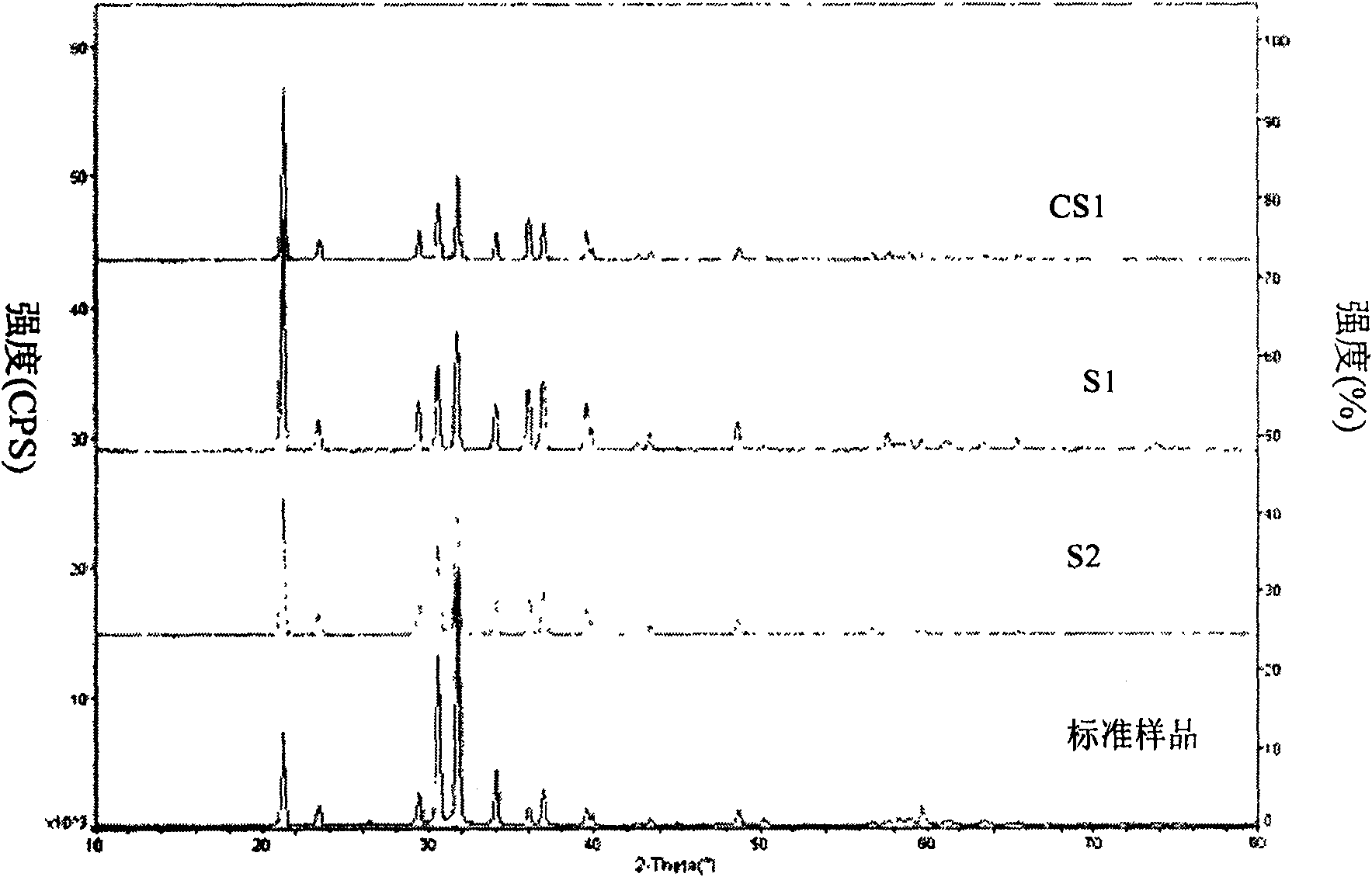
[0027] Accurately weigh 23.94 grams of analytically pure lithium hydroxide and 0.18 grams of γ-butyrolactone and dissolve them in 250 ml of deionized water to obtain a clear solution. The solution was heated to 65°C, and carbon dioxide gas in a cylinder with a pressure of 6.5 atmospheres was continuously fed into the solution at a rate of 0.05 liters / min. After 22 hours, carbon dioxide gas was stopped. The final pH value of the solution is 9. The reaction system was cooled to room temperature and settled for 24 hours, and then filtered with a sand core funnel. The obtained solid was washed three times with 45°C warm water, and then the solid product was baked in a 120°C oven for 48 hours to obtain a white powder. Lithium carbonate product S1.
Embodiment 2
[0032] This example is used to illustrate the preparation method of nanoscale lithium carbonate for batteries provided by the present invention.
[0033] Accurately weigh 42.39 grams of analytically pure lithium chloride and 1.23 grams of tributylamine and dissolve them in 400 ml of deionized water to obtain a clear solution. The solution was heated to 65°C, and carbon dioxide gas in a cylinder with a pressure of 6.5 atmospheres was continuously fed into the solution at a rate of 0.05 liters / min. After 22 hours, carbon dioxide gas was stopped. The final pH value of the solution is 9. The reaction system was cooled to room temperature and settled for 24 hours, and then filtered with a sand core funnel. The obtained solid was washed three times with 45°C warm water, and then the solid product was baked in a 120°C oven for 48 hours to obtain a white powder. Lithium carbonate product S2.
Embodiment 3
[0035] This example is used to illustrate the preparation method of nanoscale lithium carbonate for batteries provided by the present invention.
[0036] Accurately weigh 68.94 grams of analytically pure lithium nitrate and 0.49 grams of tributylamine and dissolve them in 250 ml of deionized water to obtain a clear solution. The solution was heated to 75°C, and carbon dioxide gas in a cylinder with a pressure of 6.5 atmospheres was continuously fed into the solution at a rate of 0.05 liters / min. After 22 hours, carbon dioxide gas was stopped. The final pH value of the solution is 9. The reaction system was cooled to room temperature and settled for 24 hours, then filtered with a sand core funnel, and the obtained solid was washed three times with 55°C warm water, and then the solid product was placed in a 120°C oven for 48 hours to obtain a white powder. Lithium carbonate product S3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com