Microemulsion containing matrine
A technology of matrine and microemulsion, which is applied in the field of medicine, can solve the problems of low drug concentration in target organs, low drug skin penetration rate, and short half-life of oral preparations, so as to prolong circulation time, promote drug absorption, and facilitate industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 prepares matrine microemulsion
[0022] Formula and ratio:
[0023] Matrine 1g Isopropyl myristate 6g
[0024] Tween 805g Deionized water 34g
[0025] Phospholipid 4g prepared drug-containing microemulsion 50g
[0026] The preparation method is:
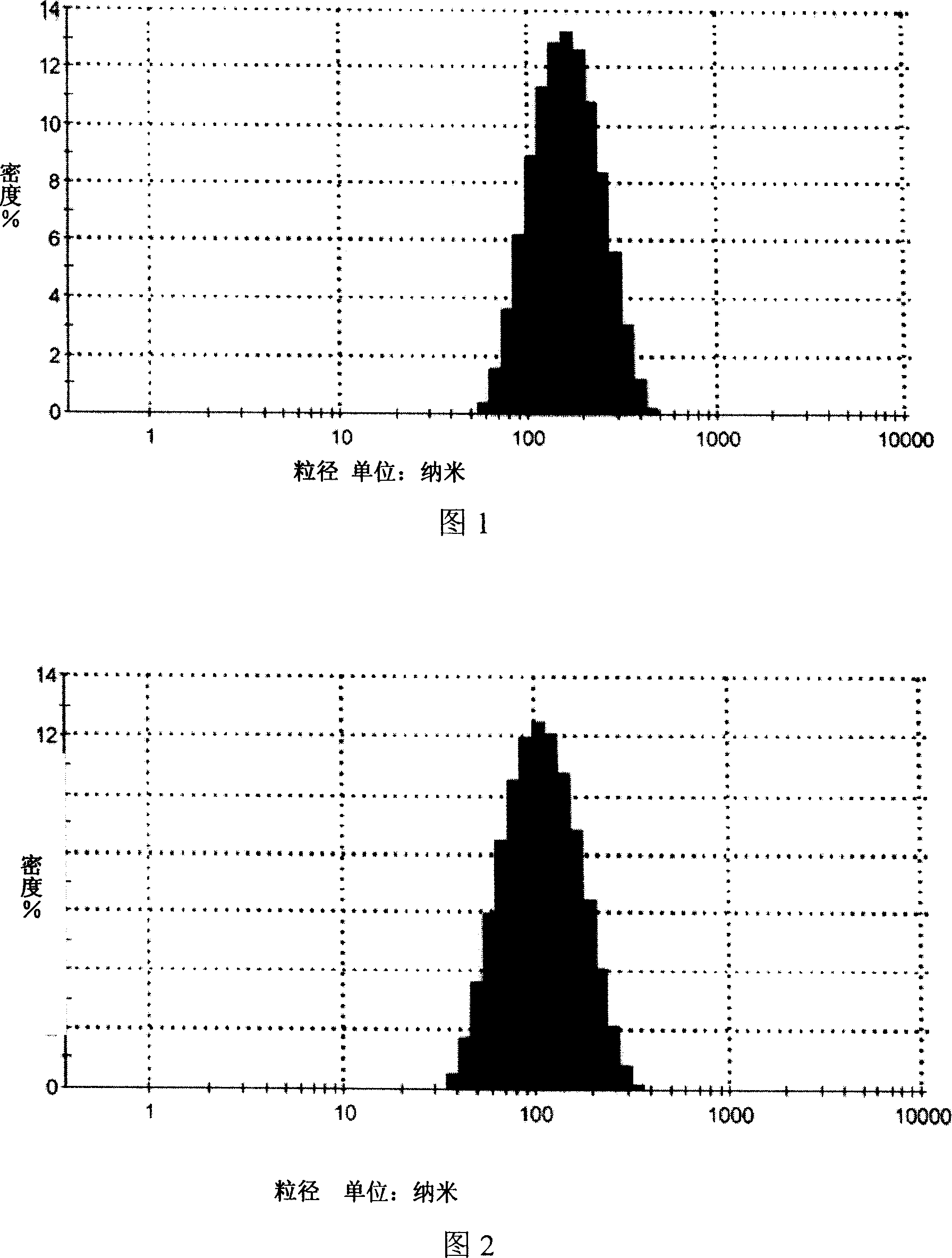
[0027] 1. High-pressure homogenization method, mixing deionized water and Tween 80 as (I) phase, mixing isopropyl myristate and phospholipids as (II) phase, dissolving matrine into (II) phase as phase (II) For the internal phase of the drug, mix the internal phase containing the drug with the phase (I) under continuous stirring, and homogenize the mixture for 3-5 times under the homogeneous condition of the primary valve pressure of 60-70 bar and the secondary valve pressure of 600-700 bar , to obtain drug-containing microemulsion, the drug-containing particle size distribution of Sophora flavescens microemulsion is detected by dynamic light scattering method, the results are shown in Figure 1, the microemulsi...
Embodiment 2
[0029] Embodiment 2 prepares matrine microemulsion
[0030] Formula and ratio:
[0031] Matrine 2g Oleic Acid 12g
[0032] Tween 206g Deionized water 50g
[0033] Caprylic acid / capric acid macrogol glyceride 10g prepared drug-containing microemulsion 80g
[0034] The preparation method is:
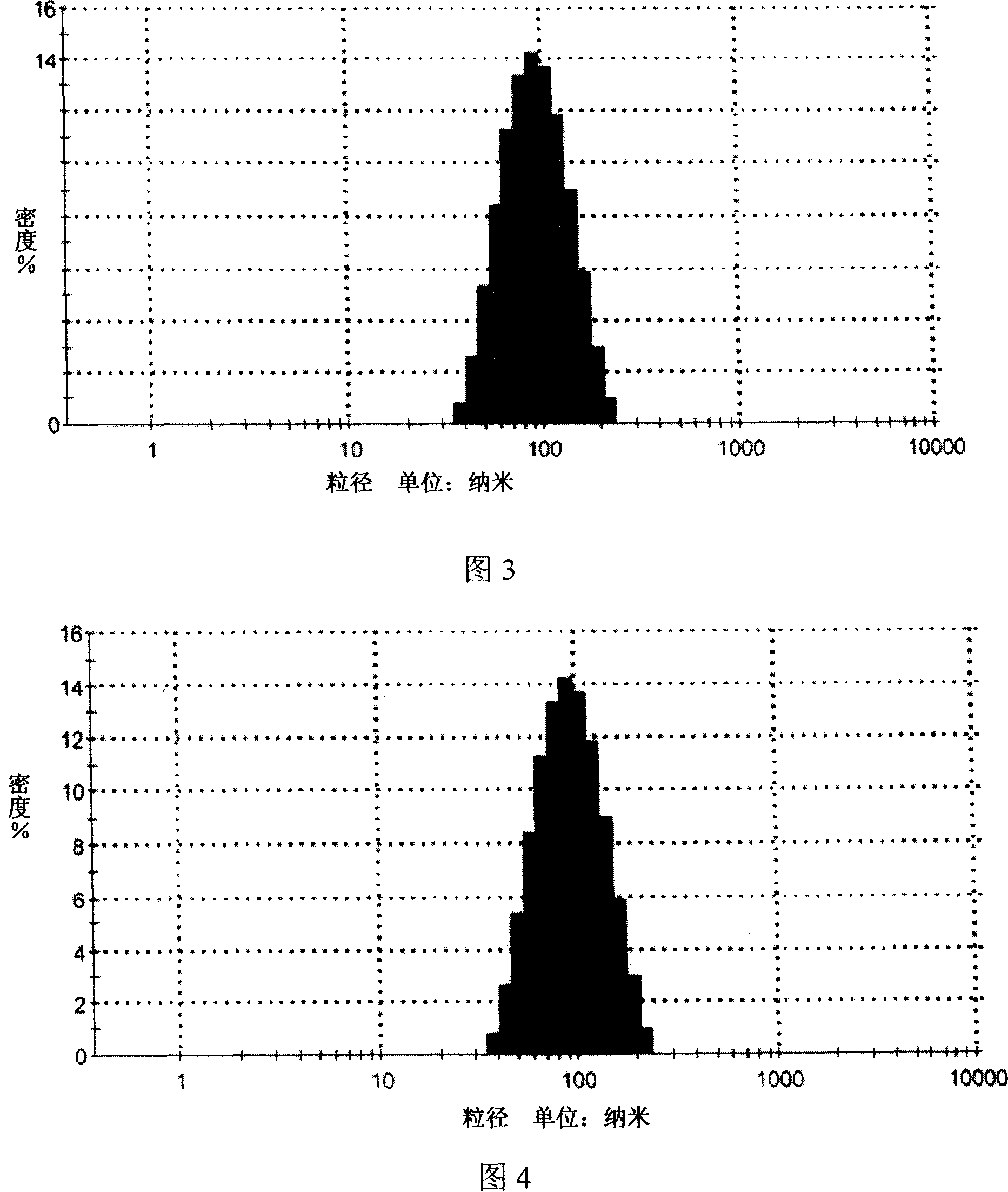
[0035] 1. Manual drop method, mix deionized water and Tween 20 as (I) phase, mix caprylic / capric macrogol glyceride and oleic acid as (II) phase, dissolve matrine into (II) In the phase, it is the drug-containing internal phase, and the drug-containing internal phase is added dropwise at a speed of 1ml / min under continuous stirring at 40°C to form a clear drug-containing microemulsion. The particle size distribution is detected by the dynamic light scattering method, and the results are shown in Figure 3. The particle size range of the microemulsion is about 100nm (90-110nm), the particle size is uniform, and the appearance is clear.
[0036] 2. Ultrasonic emulsification method, mixin...
Embodiment 3
[0037] Embodiment 3 prepares matrine microemulsion
[0038] Formula and ratio:
[0039] Matrine 4g ethyl acetate 15g
[0040] Tween 8010g deionized water 55g
[0041] 6g of phospholipids prepared 100g of drug-containing microemulsion
[0042] The preparation method is:
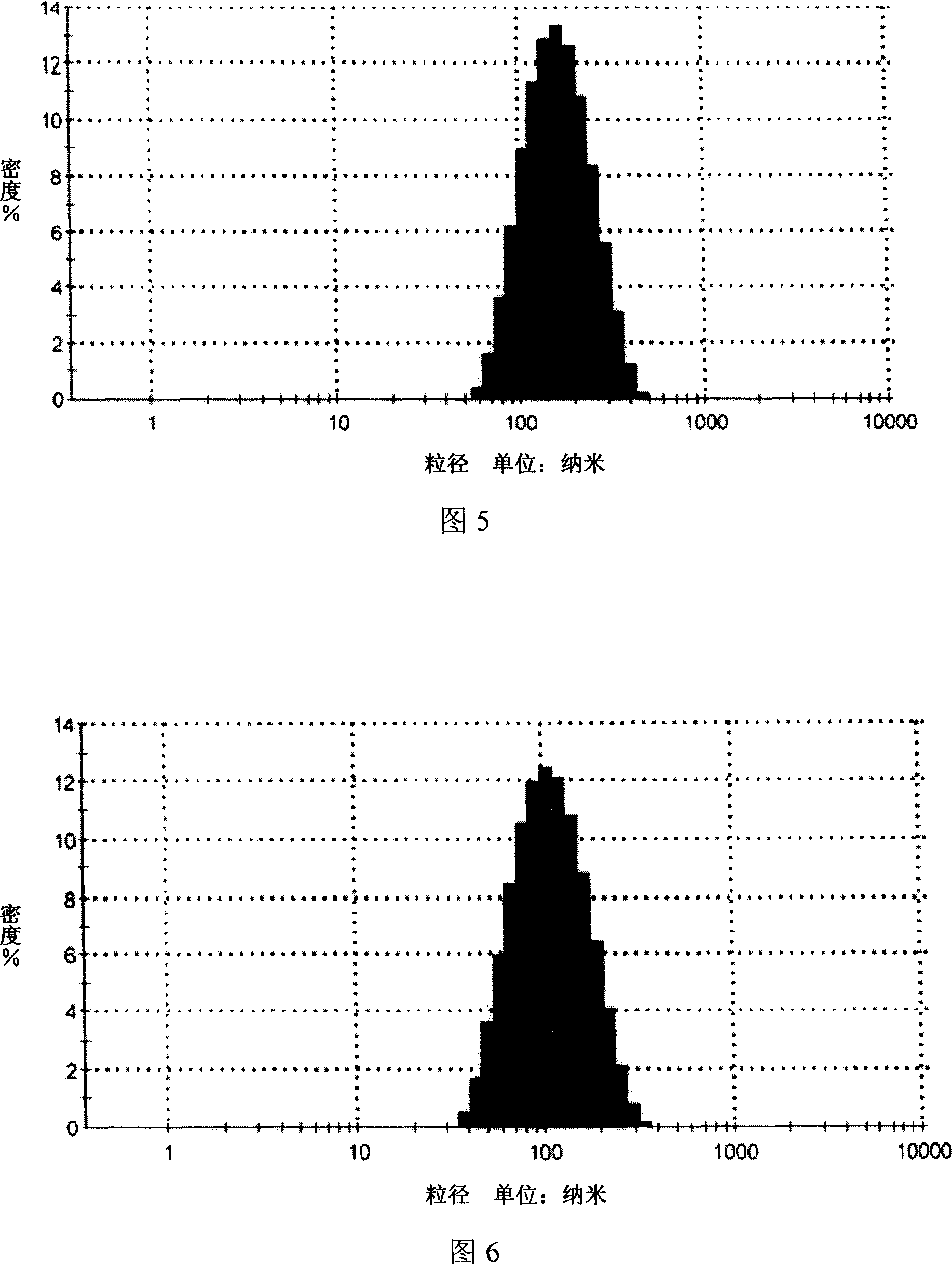
[0043] 1. Ultrasonic emulsion method, mixing deionized water and Tween 80 as (I) phase, miscible ethyl acetate and phospholipids as (II) phase, dissolving matrine into (II) phase as drug-containing phase, under continuous stirring, the drug-containing internal phase and (I) phase are miscible, and the mixture is ultrasonically emulsified for 30 min under a power of 250w to obtain a drug-containing microemulsion. The drug-containing particle size distribution of the Sophora flavescens microemulsion is dynamically The light scattering method is used for detection, and the results are shown in Figure 5. The particle size range of the microemulsion is about 100nm (90-110nm), the particle size is uniform, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com