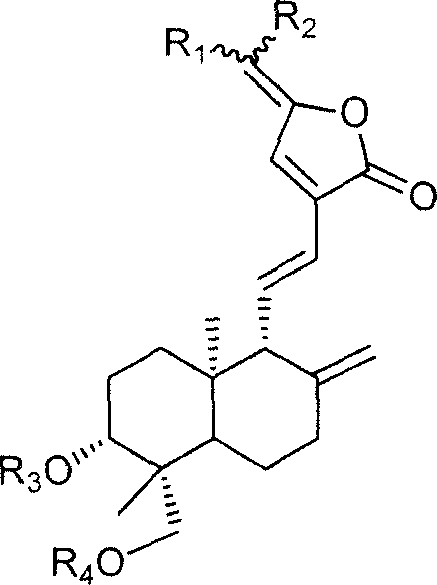
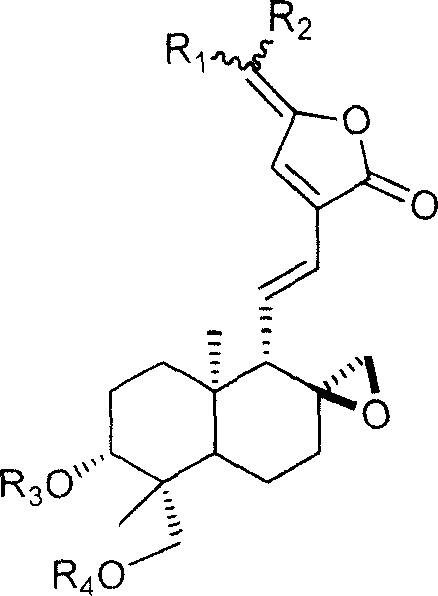
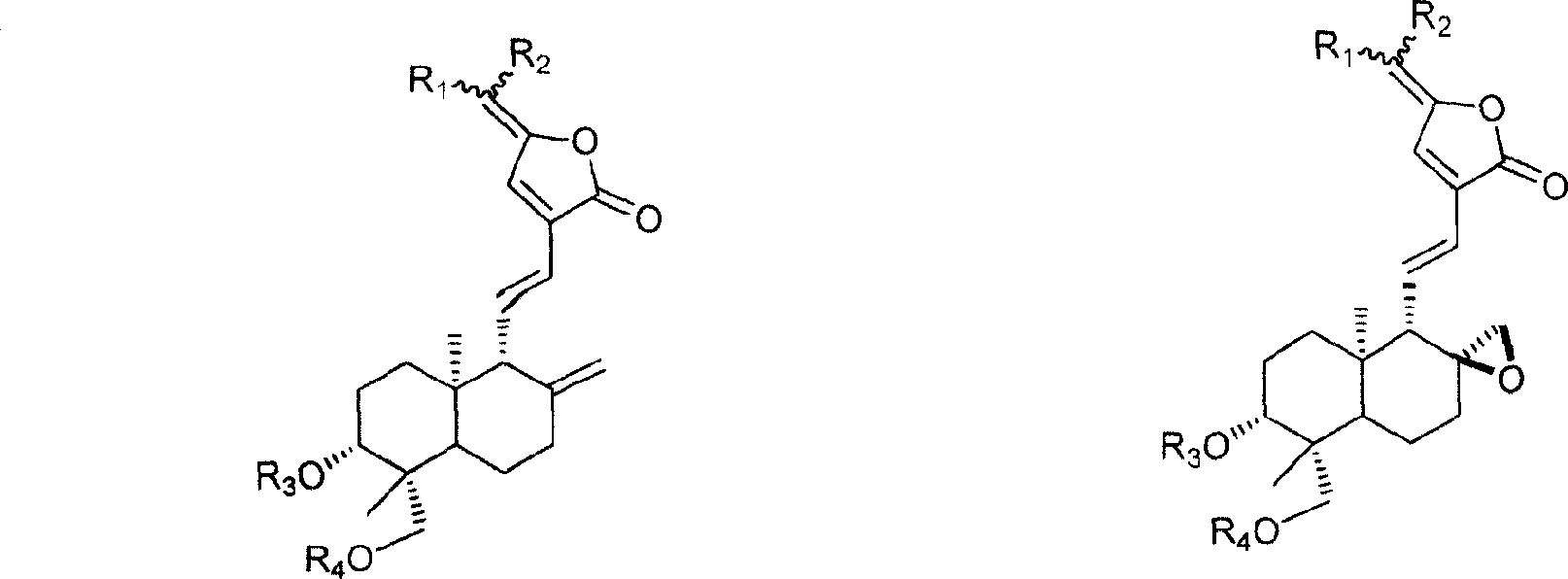
15-(14-deoxy 11,12 dehydro) andrographolide carbonic side derivate and preparation process thereof
A technology of ester carbon glycoside derivatives and andrographolide, applied in the field of carbon glycoside compounds and preparation thereof, can solve the problems such as no reports of C15-andrographolide carbon glycoside derivatives, and achieves simple and convenient preparation method, resource Rich, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: prepare R shown in general formula 1 1 =(β-D-4-chloro-4-deoxy-galactosyl-1-)-methyl, R 2 = methyl, R 3 = R 4 = Derivative when H
[0017] 14-deoxy-11, 12-dehydroandrographolide 680mg, (β-D-4-chloro-4-deoxy-galactosyl-1-)-acetone 238mg dissolved in 10ml of methanol, adding 50mg of sodium carbonate, 65 °C for 6 hours. After the reaction, add 16 ml of chloroform to dilute, add silica gel to the suction filter funnel and filter to remove the alkali in the system, concentrate the reaction system, and separate by column chromatography to obtain 414 mg of light yellow solid, which is a pair of cis-trans isomerism mixture, cis / trans = 1 / 1, yield 75%. The experimental data are as follows:
[0018] C 29 h 41 ClO 8 .IR3391, 2935, 1744, 1077, 1031cm -1 ; 1 H NMR (400MHz, DMSO, TMS): δ8.01(s, 1H), 7.80(s, 1H,), 6.76(dd, 2H, J=10.4, 15.6Hz), 6.16(dd, 2H, J=12.8 , 15.6 Hz), 5.37(w, 2H, OH), 5.19(w, 2H, OH), 5.07(w, 2H, OH), 4.78(w, 2H, OH), 4.78(s, 2H), 4.44(...
Embodiment 2
[0019] Embodiment 2: prepare R shown in general formula 2 1 = (β-D-xylosyl-1-)-methyl, R 2 = methyl, R 3 = R 4 = Derivative when H
[0020] 14-deoxy-11,12-dehydro-8,17 epoxyandrographolide 600mg, (β-D-xylosyl-1-)-acetone 190mg dissolved in 10ml ethanol, add 60mg sodium bicarbonate, 70℃ React for 6 hours. After the reaction, add 16 ml of chloroform to dilute, add silica gel to the suction filter funnel and filter to remove the alkali in the system, concentrate the reaction system, and separate by column chromatography to obtain 427 mg of light yellow solid, which is a pair of cis-trans isomerism mixture, cis / trans = 1 / 1, 82% yield. The experimental data are as follows:
[0021] C 28 h 40 o 9 .IR3410, 2933, 1748, 1038em -1 ; 1H NMR (400MHz, DMSO, TMS): δ7.92(s, 0.5H), 7.80(s, 0.5H), 6.30(m, 1H), 6.08(m, 1H), 5.02(w, 4H, OH) , 4.12(w, 1H, OH), 3.81(d, 1H, J=10.9Hz), 3.63(m, 1H), 3.38-3.17(6H, overlap), 3.16(m, 1H), 3.06(m, 1H ), 2.90(m, 1H), 2.65(m, 2H), 2.45(m, 1H),...
Embodiment 3
[0022] Example 3: Preparation of R1=(β-D-galactosyl-1-)-methyl group shown in general formula 1, R 2 = methyl, R 3 = R 4 =CH 2 time derivative
[0023] 14-deoxy-11,12-dehydro-3,19-formal andrographolide 650mg, (β-D-galactosyl-1-)-acetone 220mg dissolved in 10ml of acetonitrile, added 50mg of triethylamine, 70 °C for 6 hours. After the reaction, the reaction system was concentrated and separated by column chromatography to obtain 426 mg of light yellow solid, which was a pair of cis-trans isomerism mixture, cis / trans=1 / 1, yield 78%. The experimental data are as follows:
[0024] C 30 h 42 o 9 .IR3400, 2939, 1753, 1445, 1026cm -1 ; 1 H NMR (400MHz, CDCl 3 , TMS): δ7.41(s, 1H), 7.32(s, 1H), 6.90(m, 1H), 6.14(d, 1H, J=15.6Hz), 4.90(s, 2H), 4.78(s, 2H), 4.29(s, 2H), 4.26-3.42(14H, overlap), 2.01(s, 3H), 1.41(s, 3H), 0.92(s, 3H); 13 C NMR (100.6 MHz, CDCl 3 , TMS): δ169.7, 148.8, 147.3, 147.1, 132.6, 132.3, 127.9, 127.7, 122.9, 110.3, 88.5, 87.3, 83.1, 81.0, 80.6, 79.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com