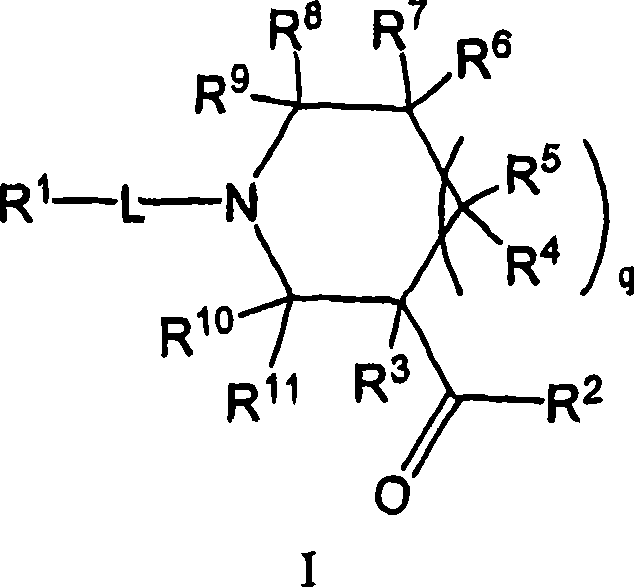
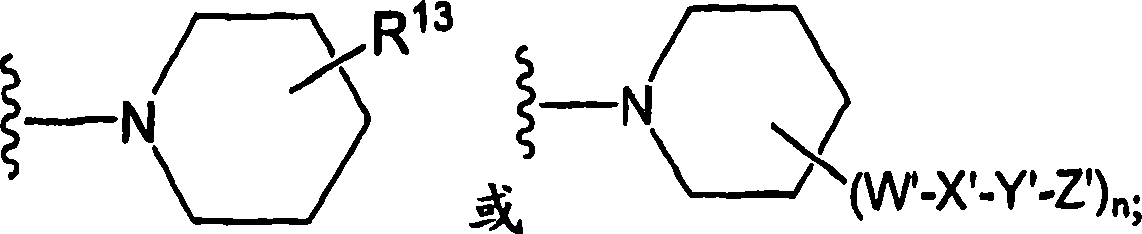
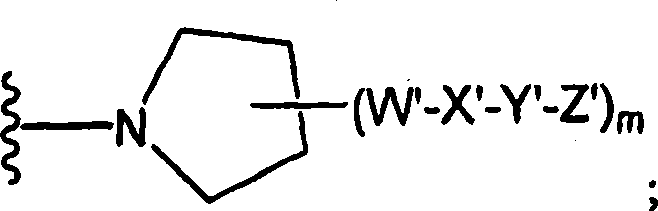Amido compounds and their use as pharmaceuticals
A technology of compounds and prodrugs, applied in the field of modulators of type 1 11-β hydroxysteroid dehydrogenase and/or mineralocorticoid receptors, capable of solving problems such as increased risk of fractures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0413] Preparation of compounds may involve protection and deprotection of various chemical groups. The need for protection and deprotection, and the selection of suitable protecting groups, are readily determined by those skilled in the art. The chemistry of protecting groups can be found in Green et al., Protective Groups in Organic Synthesis (Second Edition, Wiley & Sons, 1991), the contents of which are incorporated herein by reference in their entirety.
[0414] The reactions in the methods described herein can be carried out in suitable solvents which can be readily selected by those skilled in the art of organic synthesis. Suitable solvents can be substantially nonreactive with the starting materials (reactants), intermediates or products at the temperature at which the reaction is carried out (ie, can be a temperature ranging from the solvent's freezing point to the solvent's boiling point). A given reaction can be carried out in one solvent or a mixture of more than ...
Embodiment 1
[0476]
[0477] 3-(1-{[1-(phenylsulfonyl)piperidin-3-yl]carbonyl}pyrrolidin-3-yl)pyridine
[0478] step 1:
[0479] To 1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid (46mg, 0.2mmol), 3-pyrrolidin-3-ylpyridine (30mg, 0.20mmol), hexafluorophosphonic acid benzotriazole-1- To a solution of oxotris(dimethylamino)phosphonium salt (94 mg, 0.21 mmol) in dichloromethane (1.0 ml) was added N,N-diisopropylethylamine (46 μL, 0.26 mmol). The reaction mixture was stirred overnight at room temperature and directly purified by flash chromatography to obtain 65 mg (yield: 89%) of 3-[(3-pyridin-3-ylpyrrolidin-1-yl)carbonyl]piperidine-1 - tert-butyl carboxylate.
[0480] Step 2:
[0481] A solution of tert-butyl 3-[(3-pyridin-3-ylpyrrolidin-1-yl)carbonyl]piperidine-1-carboxylate (65 mg, 0.18 mmol) in 1.0 ml dichloromethane and 1.0 ml TFA After stirring at room temperature for 2 hours, concentration gave 88 mg (100%) of 3-[1-(piperidin-3-ylcarbonyl)pyrrolidin-3-yl]pyridine bis(trifluo...
Embodiment 2
[0485]
[0486] 3-[1-({1-[(2-nitrophenyl)sulfonyl]piperidin-3-yl}carbonyl)pyrrolidin-3-yl]pyridine
[0487] 3-[1-(Piperidin-3-ylcarbonyl)pyrrolidin-3-yl]pyridine bis(trifluoroacetic acid) salt (21mg, 0.043mmol), o-nitrobenzenesulfonyl chloride (9.5mg, 0.043mmol) and a mixture of triethylamine (21 μl, 0.151 mmol) in acetonitrile (200 μl) was stirred at room temperature for 2 hours. The reaction mixture was directly purified by HPLC to obtain 13.6 mg of the desired product (yield: 71%). LCMS: m / z 445.0 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com