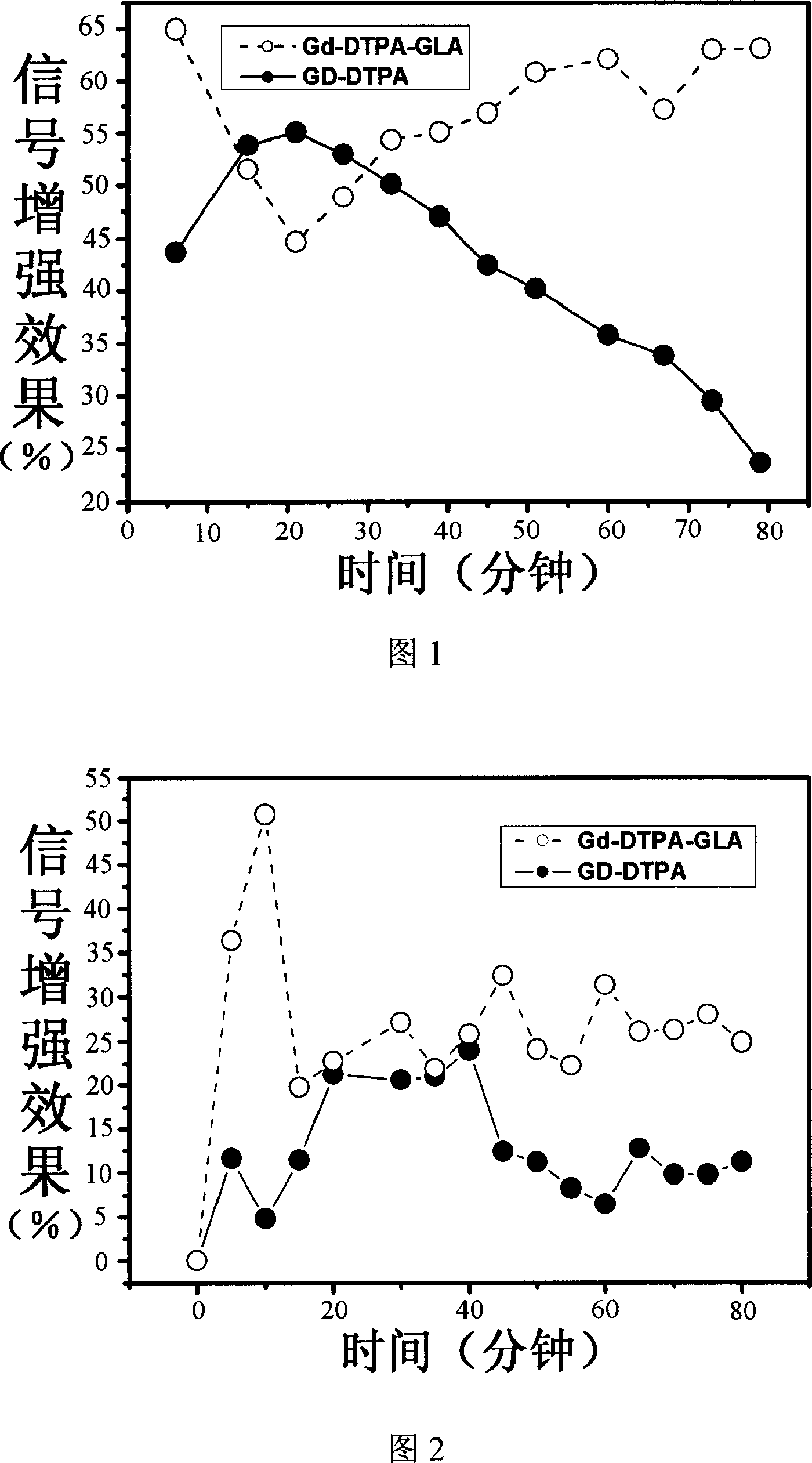
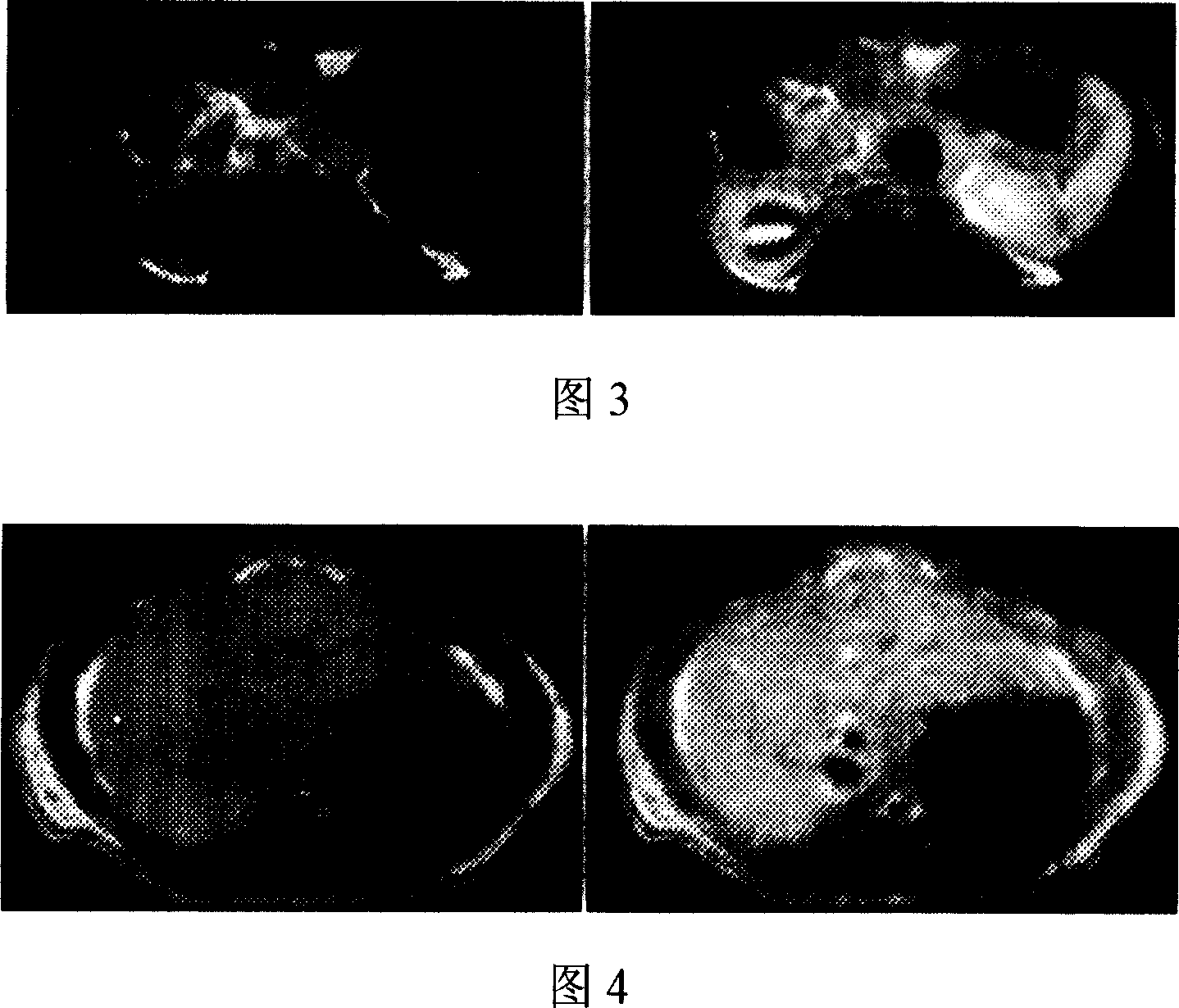
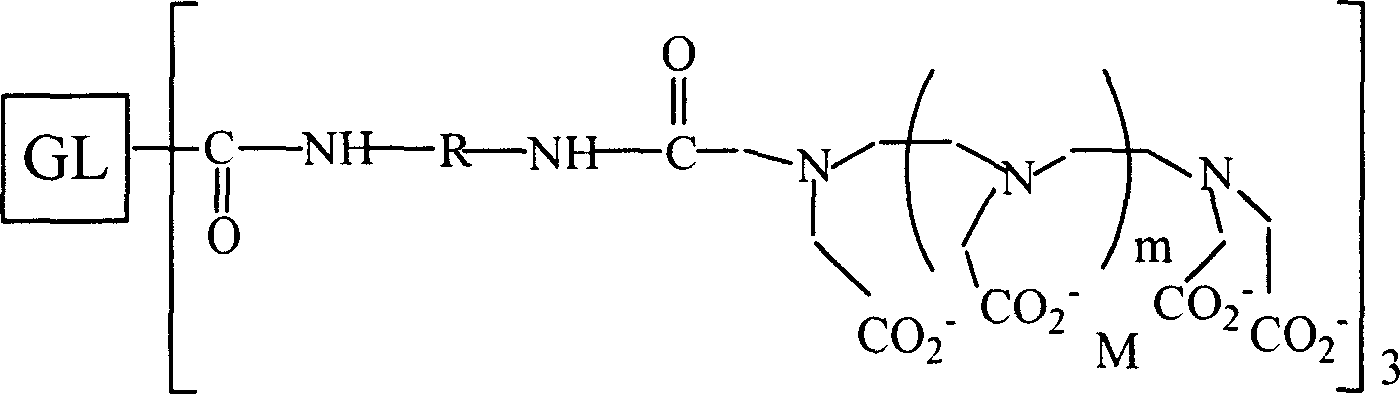
Magnetic resonace imaging contrast medium with glycyrrhizic acid as carrier
A technology of magnetic resonance imaging and glycyrrhizic acid, which is applied in the direction of nuclear magnetic resonance/magnetic resonance imaging contrast agents, preparations for in vivo tests, and pharmaceutical formulations, can solve the problems of decomposing free gadolinium during residence time, and achieve good selectivity, Effect of Improving Imaging Contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Glycyrrhizic Acid Contrast Agent for Magnetic Resonance Imaging by Gd-Gd-DTPA-GLA Modified by Ethylenediamine
[0052] a) Dissolve 8g of DTPA in a mixed solution of 10ml of acetonitrile and 11.3ml of triethylamine, raise the temperature to 55°C, after DTPA is completely dissolved, add 2.87g of DCC and 1.66g of HONSu at the same time, stir at room temperature for 1.5h, and filter to obtain DTPA-ONSu solution.
[0053] b) Dissolve 5.06g of glycyrrhizic acid in 40ml of THF at -15°C, then add 3.81g of DCC, stir for 30min, add 2.13g of HONSu, stir at -15°C for 3h, continue stirring at room temperature for 24h, and filter out bicyclic The solution obtained after hexylurea was poured into 100 ml of anhydrous ether, the white solid was collected, washed three times with absolute ethanol and anhydrous ether, and dried in vacuum to obtain GL-ONSu.
[0054] c) Dissolve 2.23g of GL-ONSu obtained in step b) in 12ml of DMF, slowly add dropwise to 28ml of DMF solution ...
Embodiment 2
[0058] Preparation of Glycyrrhizic Acid as a Magnetic Resonance Imaging Contrast Agent Modified by Ethylenediamine Diethylenetriaminepentaacetate Manganese Complex
[0059] a) Same as a in Example 1.
[0060] b) Same as b in Example 1.
[0061] c) Same as c in Example 1.
[0062] d) Same as d in Example 1.
[0063] e) Dissolve 0.52g of DTPA-GLA in step d) in 10ml of deionized water, add 3.75ml of 0.2mol / L MnCl 2 Solution, adjust the pH value to 6.5 with 1mol / L NaOH, dialyze, exchange the aqueous solution outside the dialysis bag until its longitudinal relaxation time T 1 More than 3000ms, lyophilized to obtain the magnetic resonance imaging contrast agent of manganese diethylenetriaminepentaacetate complex modified by glycyrrhizic acid through ethylenediamine.
Embodiment 3
[0065] Preparation of Glycyrrhizic Acid as Magnetic Resonance Imaging Contrast Agent by Ethylenediamine Modified Gadolinium EDTA Complex
[0066] a) Dissolve 5.93g of EDTA in a mixed solution of 10ml of acetonitrile and 11.3ml of triethylamine, raise the temperature to 55°C, after the EDTA is completely dissolved, add 2.87g of DCC and 1.66g of HONSu at the same time, stir at room temperature for 1.5h, and filter to obtain EDTA- ONSu solution.
[0067] b) Same as b in Example 1.
[0068] c) Same as c in Example 1.
[0069] d) Dissolve 1 g of GLA in step c) in 25 ml of deionized water, add 9.7 ml of EDTA-ONSu solution in step a, adjust pH=10 with 6 mol / L NaOH, stir at room temperature for 24 h, remove acetonitrile by rotary evaporation, and dialyze After 5 days, the solution in the osmotic bag was rotary evaporated at 55° C. to a small volume, and freeze-dried to obtain the glycyrrhizic acid-modified EDTA ligand. .
[0070] e) Dissolve 0.44g of the glycyrrhizic acid-modified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com