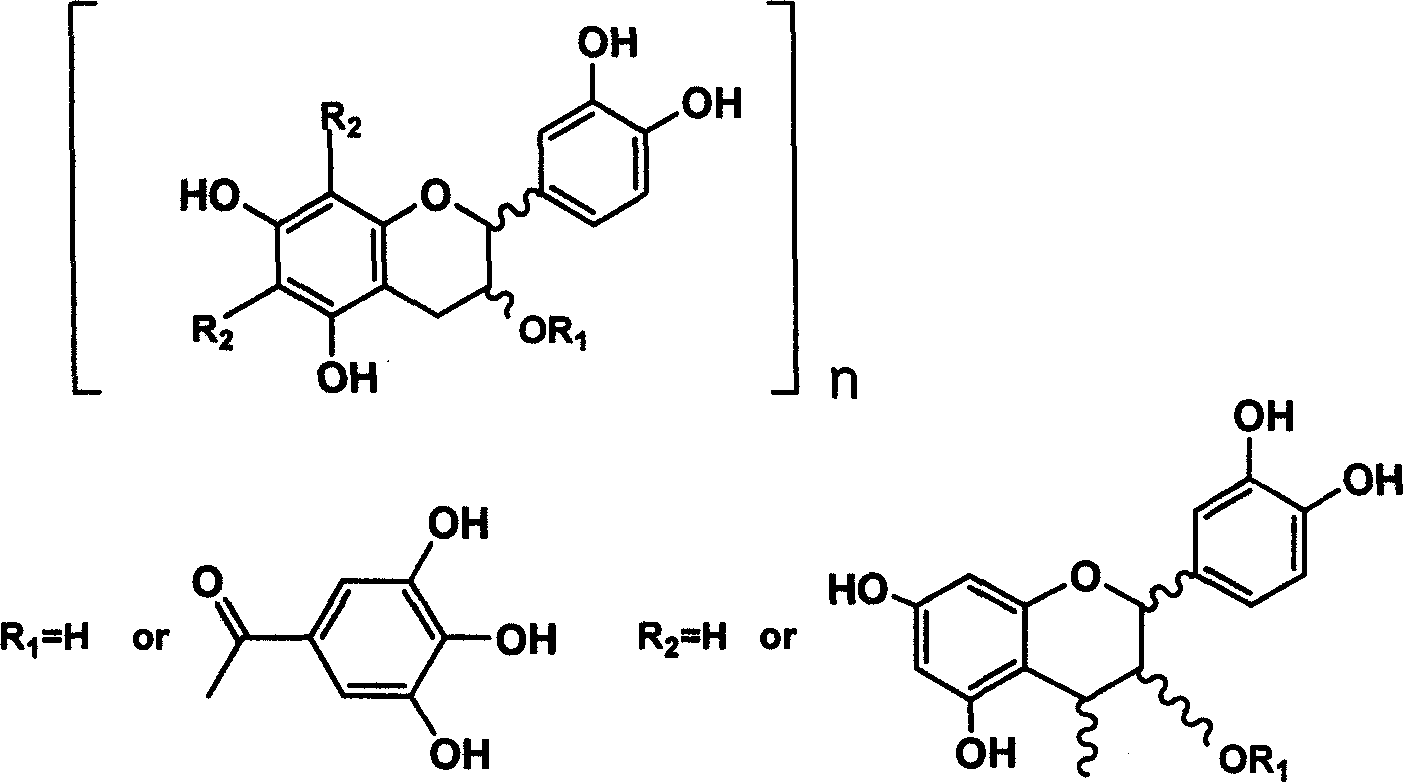
Method of preparing oligomeric proanthocyanidins
A technology of proanthocyanidins and oligomers, which is applied in the field of preparation of oligomer proanthocyanidins, can solve the problems of uneconomical production, instability, and easy decomposition, and achieve the effects of increased production, low production cost, and high antioxidant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of proanthocyanidin high-polymerization extract: weigh 250.0 g of grape seeds, crush them, pass through a 20-mesh sieve, and place them in a 100-ml beaker. Add 250ml of petroleum ether to soak for 24 hours, filter, and soak the residue three times with new petroleum ether. Add 300ml of 60% (v / v) ethanol solution to the grape seed powder after degreasing, extract at 30° for 30min, extract three times, wash the final residue twice, collect the extract and washing solution, distill under reduced pressure at ≤ 37°C, and concentrate to The specific gravity of the solution is between 1.1-1.2, and 350ml of concentrated solution is obtained. Add 200ml of ethanol to the concentrated solution to remove impurities such as protein and polysaccharide by precipitation, and repeat three times. The filtrate was distilled and concentrated under reduced pressure until the specific gravity of the solution was between 1.0-1.05 to obtain 300 ml of a concentrated solution. Add ...
Embodiment 2
[0022] Take 10ml of the proanthocyanidin high-polymerization extract prepared in Example 1, add 0.4ml of glacial acetic acid, shake well, and react in a constant temperature water bath at 60°C for 30min. The average degree of polymerization of the hydrolyzate was 3.78 by sampling analysis.
Embodiment 3
[0024] Take 10ml of the proanthocyanidin high-polymerization extract prepared in Example 1, add 0.2ml of formic acid, shake well, and react in a constant temperature water bath at 50°C for 40min. The average degree of polymerization of the hydrolyzate was 4.47 in sampling analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com