Engineering bacterium capable of producing anthracene ring antibiotics and application of the same
A technology of engineering bacteria and anthracyclines, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Cloning and sequencing of dnmU and dnmV linked genes of Streptomyces coelicolor SIPI-1482
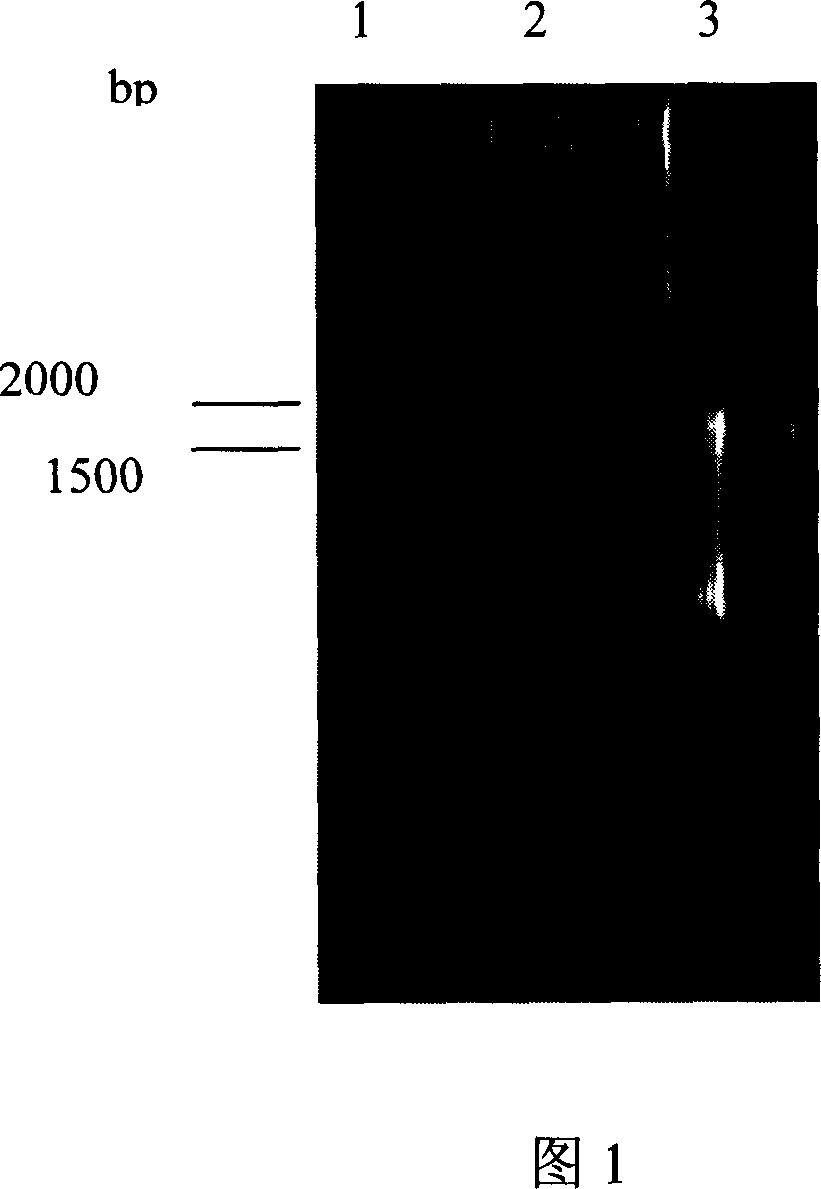
[0048] dnmU in Streptomyces coelicolor is linked to and upstream of the dnmV gene. In order to achieve directional blocking of the dnmV gene, primers were first designed according to the published corresponding sequence of S. peucetius (Accession Number AF006633), respectively dnmUV1: 5'-AA CTG CAG GAGCGAAGTGGCGTTG-3', containing PstI site; dnmUV2: 5'-GG GAATTC TCGTCGGAAGCCTGTG-3' with EcoRI site. The PCR reaction conditions were as follows: denaturation at 97°C for 5 minutes, adding Taq enzyme, 1 minute at 95°C, 1 minute at 61°C, 3 minutes at 72°C, and extension at 72°C for 10 minutes after 30 cycles. The dnmU and dnmV linkage fragments were amplified using the total genome of Streptomyces coelicolor SIPI-1482 as a template, and the full length of the PCR product was 1664bp (as shown in Figure 1). The dnmU and dnmV linked fragment PCR products were ligated to pUC...
Embodiment 2
[0049] Example 2 Construction, Transformation and Blocker Screening of Double Crossover Plasmids
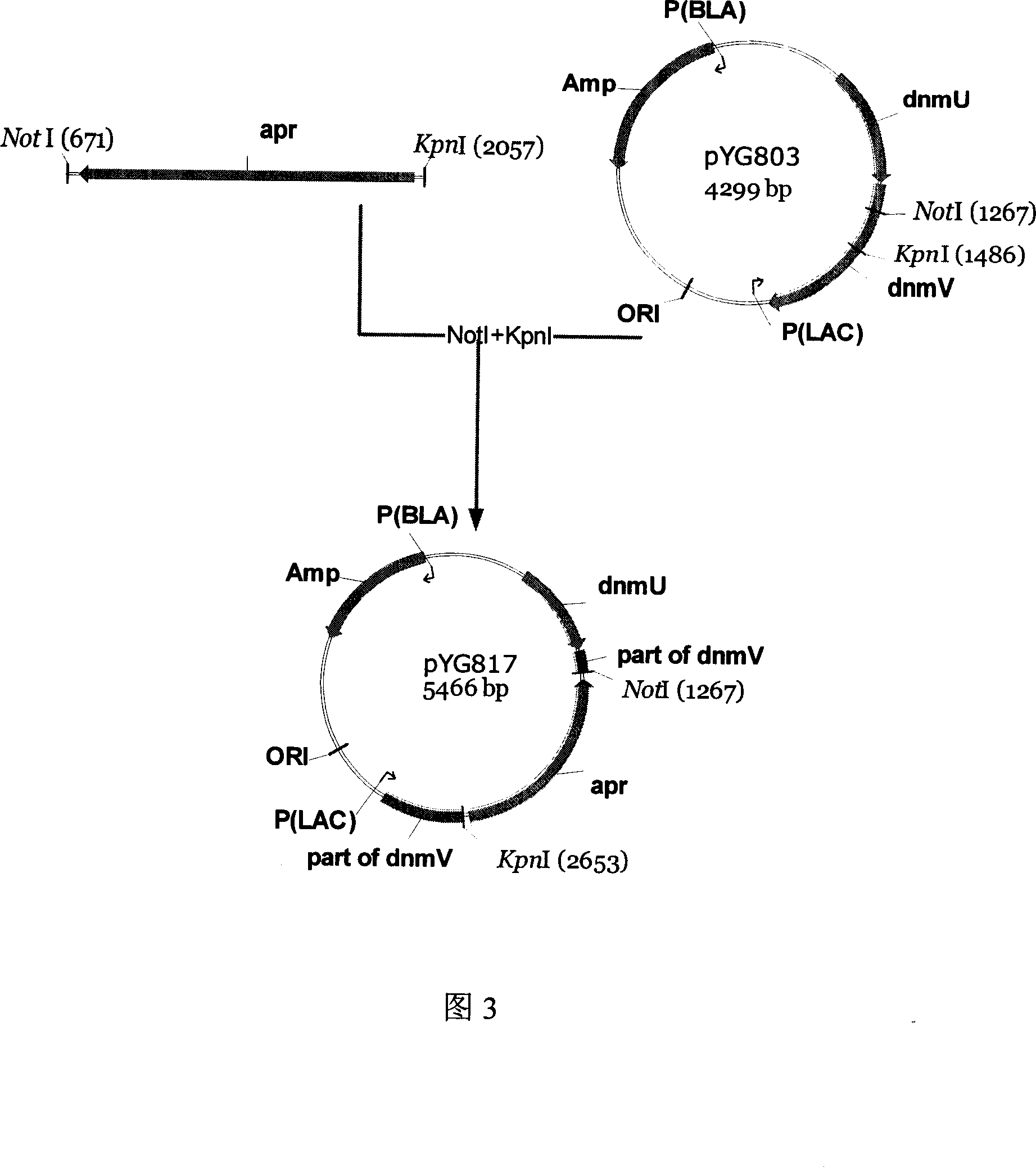
[0050] The plasmid pYG803 (4299bp) prepared in Example 1 was digested with KpnI and NotI, and a 4080bp fragment was recovered. Combine this fragment with the apramycin resistance gene fragment (published in GenBank, about 1.3kb, Accession number: AY072040, AY216678, AJ438947, AJ566337) that was also digested with KpnI and NotI and use T4 ligase at 16°C After 4 hours of ligation, E.coliDH5a was transformed. Apramycin and ampicillin were used as selection markers to jointly screen the target clones, pick transformants, extract plasmids for enzyme digestion verification, and successfully construct the double-crossover plasmid pYG817 (5466bp) (as shown in Figure 3).
[0051] Alkaline denaturation and single-strandization of the obtained plasmid, that is, 110μl plasmid dilution solution system, the concentration is about 50ug / ml, 1:1 adding 0.4M NaOH to make the final concentration 0...
Embodiment 3
[0052] Example 3 Screening of double-exchange engineering bacteria
[0053] The spores of the regenerated colonies were inoculated into 20 ml of YMB medium, cultured and collected, and their chromosomes were extracted. According to the apramycin resistance gene sequence (Accession number: AY072040, AY216678, AJ438947, AJ566337) published in GenBank, its homology was compared, and primers were designed for regions with higher homology: apr1: 5'-GATGCAGGAAGATCAACG- 3'; apr2: 5'-AGGTCTGGACGACGAGC-3'. The genomic DNA of the regenerating bacteria and the genomic DNA of Streptomyces coelicolor SIPI-1482 (control strain) were respectively used as templates for PCR amplification. As a result, a fragment of about 500 bp inside the apramycin resistance gene could be amplified from the genome of the regenerating bacteria. However, no fragments could be obtained from the genome of the control strain (as shown in FIG. 4 ).
[0054] Also use the primers dnmV1 on both sides of the dnmV gen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com