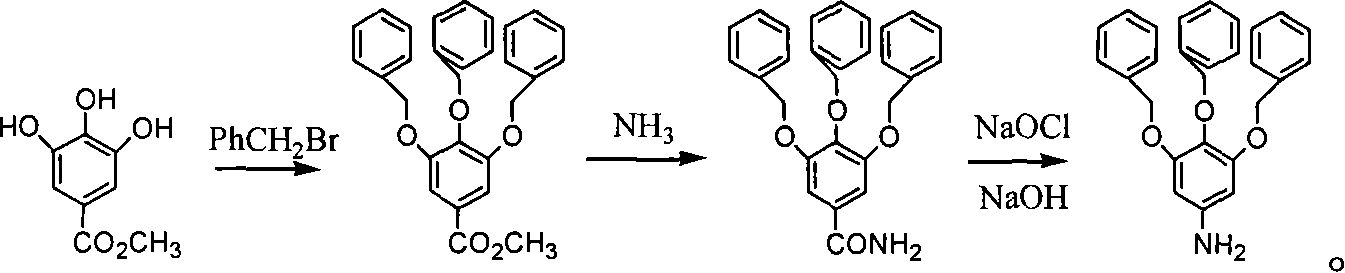
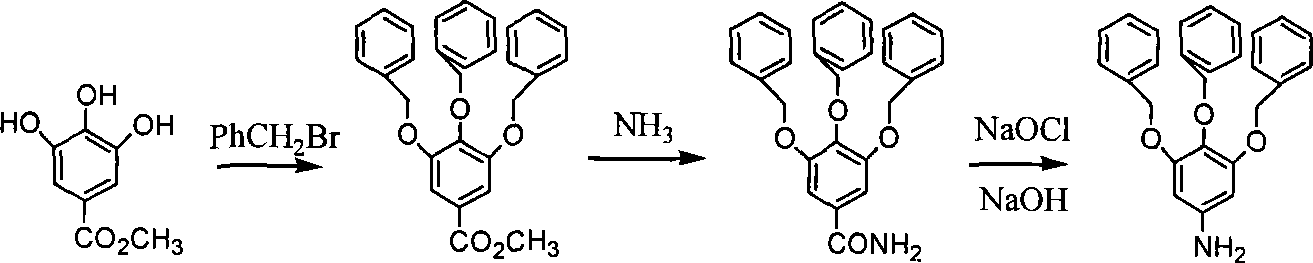
Chemical synthesis of 3,4,5-trioxyaniline
A technology of tribenzyloxyaniline and tribenzyloxybenzamide, which is applied in 3 fields, can solve the problems of low reduction efficiency and limitations, and achieve the effects of high reduction yield, low price and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: 3,4,5-tribenzyloxymethyl benzoate: add methyl trihydroxybenzoate 1.84g (0.01mol), potassium carbonate 5.52g (0.04 mol), add 40 mL of solvent N, N-dimethylformamide in a nitrogen atmosphere, then slowly add 4 mL of benzyl bromide (0.034 mol) dropwise at an oil bath temperature of 55 ° C, stir it magnetically for 24 h, and pour it rapidly into cold water, filter to remove the solvent, and put the precipitate in a vacuum drying oven to dry overnight to obtain a light yellow crude product, which is finally reconstituted with a mixed solvent of ethyl acetate:petroleum ether (boiling range 60-90°C) with a volume ratio of 1:2 Crystallization of the crude product gave the target product as colorless crystals in a yield of 91%.
Embodiment 2
[0015] Example 2: Methyl 3,4,5-tribenzyloxybenzoate: except that acetone was used instead of N,N-dimethylformamide, other operating conditions were the same as in Example 1, and colorless crystals 3,4 were obtained. , 3.45 g of methyl 5-tribenzyloxybenzoate, the yield was 76%.
Embodiment 3
[0016] Example 3: 3,4,5-tribenzyloxybenzamide: add 1.39g (3mmol) of 3,4,5-tribenzyloxymethylbenzoate into a 250mL three-necked flask, and then add 200mL ethylene glycol solvent, the temperature gradually increased to 100°C with magnetic stirring, ammonia gas was introduced into it, the reaction time was 30h, and it was allowed to stand overnight, and a precipitate was precipitated, filtered and washed with water for 3 times to obtain a needle-like white solid, which was dried and weighed 1.12 g, the yield is 83.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com