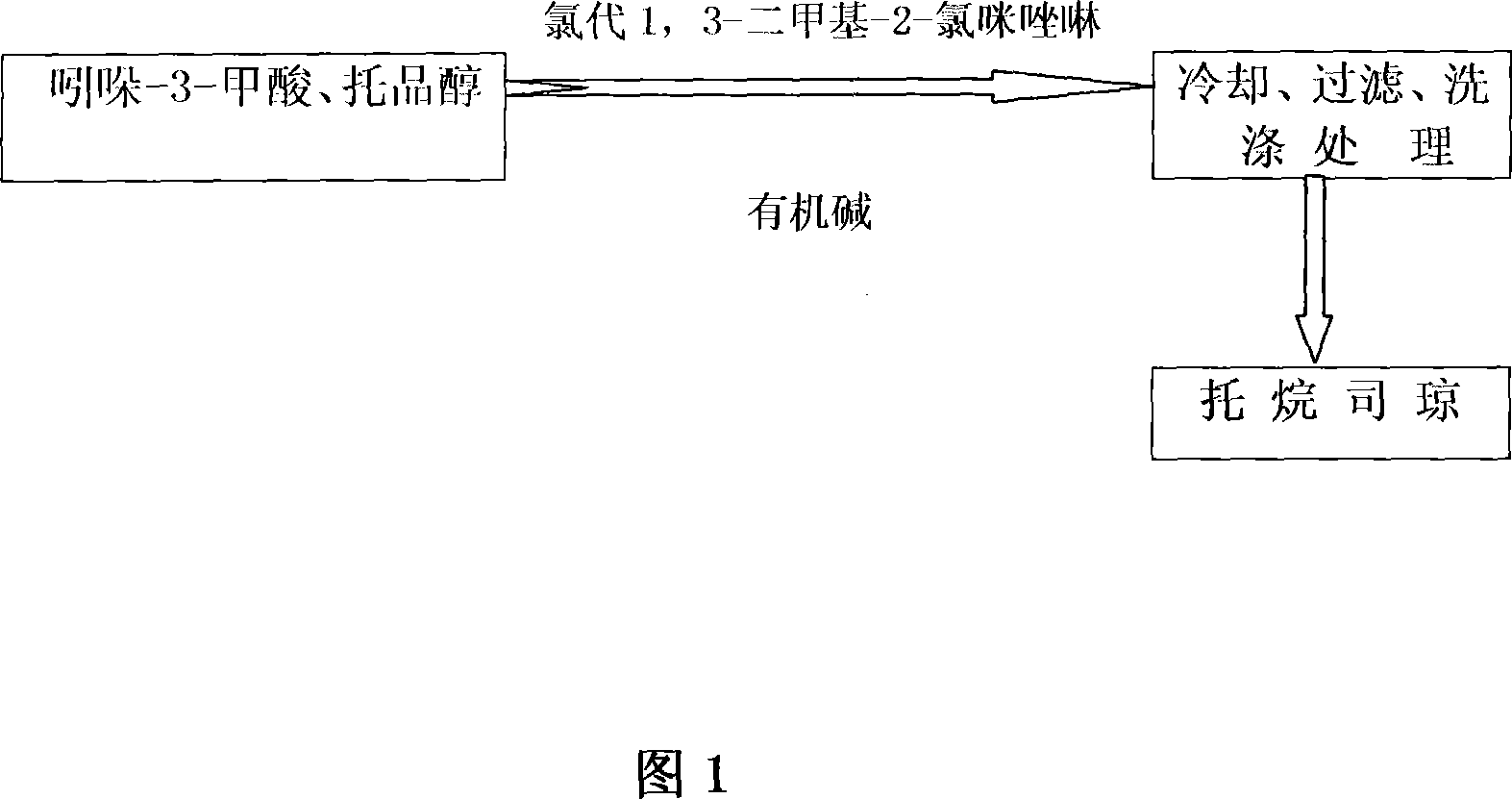
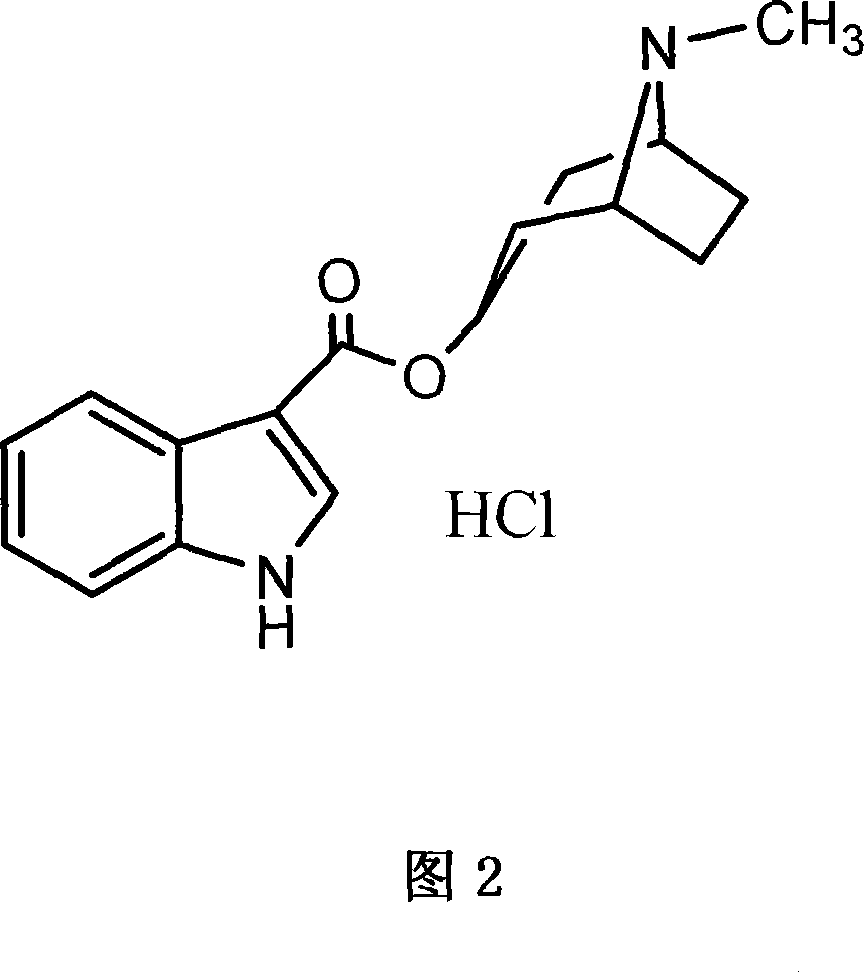
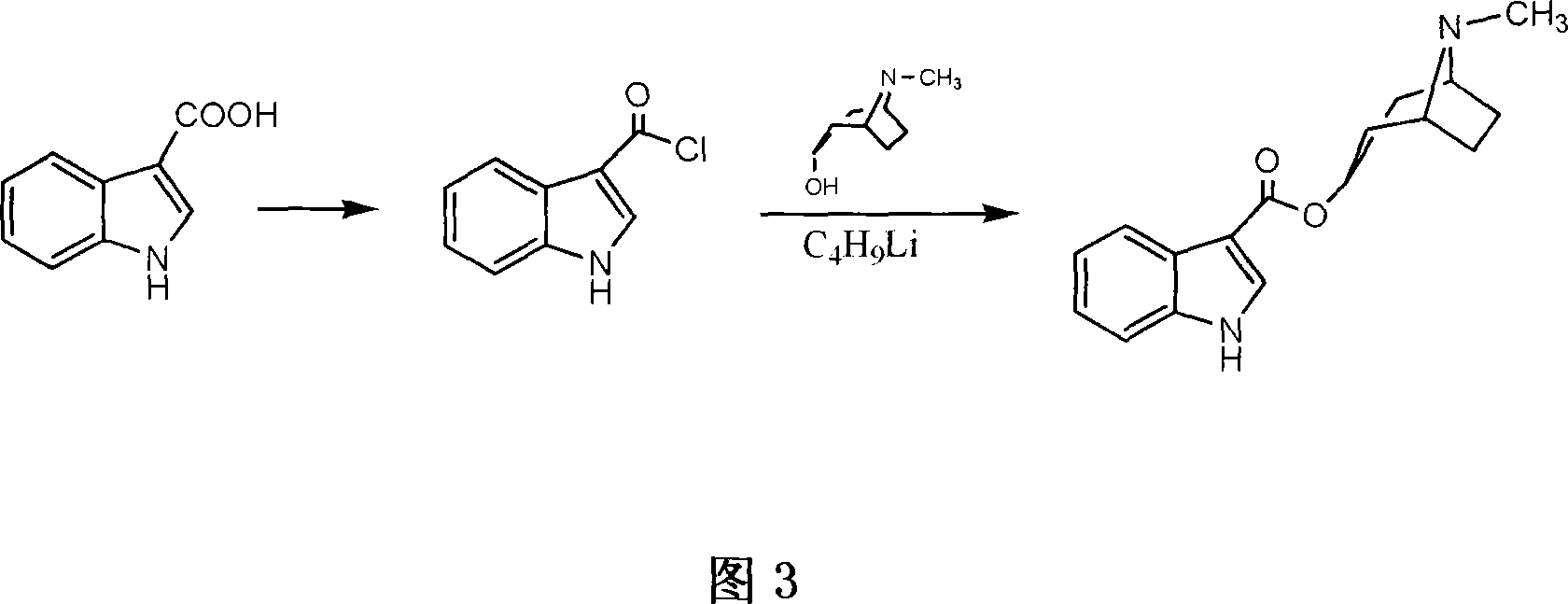
Process of preparing troipisetron
A preparation process, the technology of tropisetron, which is applied in the field of preparation process of antiemetic drug tropisetron, can solve the problems such as the inevitable by-product hydrogen chloride gas generation, and achieve the effect of simple operation, high purity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Indole-3-carboxylic acid (16.1 g, 0.1 mol), chloro-1,3-dimethyl-2-chloroimidazoline (11.4 g, 0.1 mol), tropinol (14.1 g, 0.1 mol) were dissolved in In ethyl acetate (200 ml), triethylamine (10.1 g, 0.1 mol) was added dropwise with stirring, and reacted at room temperature for 12 hours. Add water (60 ml), adjust pH=9-10 with 15% sodium hydroxide, wash with water until neutral, and dry. Concentrate to obtain light yellow solid product, add absolute ethanol (300 milliliters), after dissolving, add concentrated hydrochloric acid under cooling, make pH=2~3, carry out vacuum distillation to obtain tropisetron hydrochloride crude product, filter, anhydrous Ethanol was recrystallized to obtain 24.3 g of a white crystalline product (purity determined by liquid chromatography was greater than 99.76%), yield 76.1%; melting point: 283-285° C., and the spectrum of the product was consistent with literature reports.
Embodiment 2
[0026] Indole-3-carboxylic acid (16.1 g, 0.1 mol), chloro-1,3-dimethyl-2-chloroimidazoline (13.7 g, 0.12 mol), tropinol (14.1 g, 0.1 mol) were dissolved in In ethyl acetate (200 ml), triethylamine (15.2 g, 0.15 mol) was added dropwise with stirring, and reacted at room temperature for 18 hours. Add water (60 ml), adjust pH=9-10 with 15% sodium hydroxide, wash with water until neutral, and dry. Concentrate to obtain light yellow solid product, add absolute ethanol (300 milliliters), after dissolving, add concentrated hydrochloric acid under cooling, make pH=2~3, carry out vacuum distillation to obtain tropisetron hydrochloride crude product, filter, anhydrous Ethanol was recrystallized to obtain 25 g of a white crystalline product (purity determined by liquid chromatography was greater than 99.84%), with a yield of 78.3%; melting point: 283-285°C.
Embodiment 3
[0028] Indole-3-carboxylic acid (16.1 g, 0.1 mol), chloro-1,3-dimethyl-2-chloroimidazoline (11.4 g, 0.1 mol), tropinol (14.1 g, 0.1 mol) were dissolved in Pyridine (7.9 g, 0.1 mol) was added dropwise into ethyl acetate (200 ml) with stirring, and reacted at room temperature for 12 hours. Add water (60 ml), adjust pH=9-10 with 15% sodium hydroxide, wash with water until neutral, and dry. Concentrate to obtain light yellow solid product, add absolute ethanol (300 milliliters), after dissolving, add concentrated hydrochloric acid under cooling, make pH=2~3, carry out vacuum distillation to obtain tropisetron hydrochloride crude product, filter, anhydrous Ethanol was recrystallized to obtain 21.7 g of a white crystalline product (purity determined by liquid chromatography was greater than 99.25%), with a yield of 68.1%; melting point: 283-285°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com