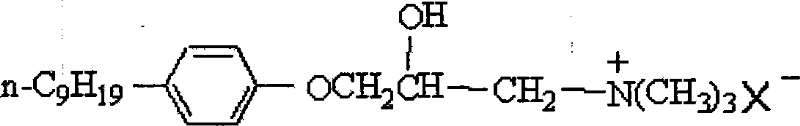
Preparation method of 3- -(P-Nonyl)Phenoxy-2- Hydroxyproyl Trimethyl Ammonium Halide
A technology of hydroxypropyltrimethylammonium halide and nonylphenoxy, which is applied in the field of surfactant preparation, can solve problems that have not been reported, and achieve the effects of improving surface activity, easy production, and good control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
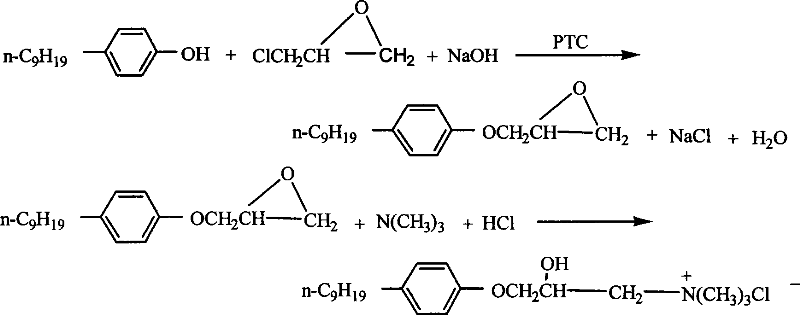
[0036] The preparation method of 3-p-nonylphenoxy group-2-hydroxypropyltrimethylammonium halide comprises the steps:
[0037] (1) Synthesis of p-nonylphenyl glycidyl ether
[0038] Add 0.1 mol (about 22 g) of p-nonylphenol, 50 ml of toluene, and about 1 g of tetrabutylammonium bromide into a 250 ml four-neck flask and stir to dissolve; weigh 0.1 mol (4.0 g) of NaOH and dissolve in about 4 ml of distilled water Add it dropwise to a four-neck bottle, stir for 3 minutes and heat it with warm water. When the temperature rises to 50°C±1°C, add 0.2 moles of epichlorohydrin (about 16ml) dropwise, and finish adding in 15min to 16min, then raise the temperature to 70°C ℃±1℃, 5000 rpm vigorously stirred and reacted for 4h; after the reaction was completed, the product solution was slightly cooled and washed with 50℃ warm water in a separatory funnel for 2 to 3 times, then the upper organic phase was taken out and transferred to an Erlenmeyer flask , add 5 g of anhydrous sodium sulfate,...
Embodiment 2
[0044] The preparation method of 3-p-nonylphenoxy group-2-hydroxypropyltrimethylammonium halide comprises the steps:
[0045] (1) Synthesis of p-nonylphenyl glycidyl ether
[0046] Add p-nonylphenol, toluene, and tetrabutylammonium bromide into the four-necked bottle, stir to dissolve it; weigh NaOH, dissolve it in distilled water, drop it into the four-necked bottle, heat it with warm water after stirring for 2 minutes, when the temperature After rising to 50°C±1°C, add epichlorohydrin dropwise for 15min to 16min, then raise the temperature to 70°C±1°C, stir and react at 4000 rpm for 5h; after the reaction is completed, cool the product solution and use a 50°C Warm water was washed 3 times in a separatory funnel, then the upper organic phase was taken out, transferred to an Erlenmeyer flask, anhydrous sodium sulfate of 1 / 10 of the volume weight of the organic phase was added, and dried overnight; then the liquid was transferred to a pear-shaped flask, Distill toluene and epi...
Embodiment 3
[0054] The preparation method of 3-p-nonylphenoxy group-2-hydroxypropyltrimethylammonium halide comprises the steps:
[0055] (1) Synthesis of p-nonylphenyl glycidyl ether
[0056] Add p-nonylphenol, toluene, and tetrabutylammonium bromide into the four-necked bottle, stir to dissolve it; weigh NaOH, dissolve it in distilled water, drop it into the four-necked bottle, stir for 5 minutes, and heat it with warm water. After rising to 50°C±1°C, add epichlorohydrin dropwise, and finish adding in 15min~16min, then raise the temperature to 70°C±1°C, stir and react at 4500 rpm for 4.5h; Wash twice with warm water in a separatory funnel, then take out the upper organic phase, transfer it to an Erlenmeyer flask, add anhydrous sodium sulfate 1 / 9 of the volume weight of the organic phase, and dry it overnight; then transfer the liquid to a pear-shaped flask , use a rotary evaporator to distill toluene and epichlorohydrin to obtain colorless p-nonylphenyl glycidyl ether;
[0057] Wherei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com