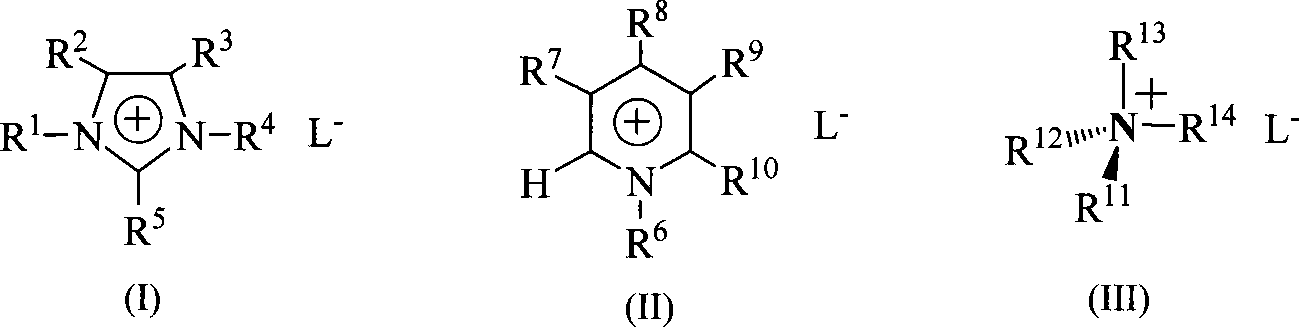
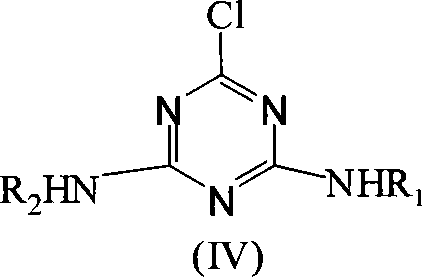
Method for synthesizing 4,6-disubstituted amido-1,3,5-triazine derivative
A synthetic method and a two-substitution technology, applied in the field of herbicides, can solve problems such as density differences, and achieve the effects of fast reaction speed, mild conditions, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Synthesis of 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine
[0036] In a 250mL three-necked flask, add 100g of ionic liquid [C 4 mim] BF 4 , cooled the temperature to -20°C with an ice-salt bath, quickly added 6.05g (0.0328mol) of cyanuric chloride, and after stirring evenly, added dropwise 2.74g of 70% isopropylamine solution (containing 1.92g of isopropylamine, 0.0325mol). After the dropwise addition, at this temperature, 4.3 g of 30% sodium hydroxide solution (containing 1.3 g of sodium hydroxide, 0.0325 mol) was added dropwise. After the dropwise addition was completed, stirring was continued for 10 min.
[0037] Gradually raise the temperature to 15°C, add dropwise 2.3g of 65% ethylamine solution (containing 1.5g ethylamine, 0.0333mol), after the dropwise addition, at this temperature, add dropwise 4.48g of 30% sodium hydroxide solution (containing sodium hydroxide 1.344g, 0.0336mol). After the dropwise addition was completed, the stirrin...
Embodiment 2
[0040] Example 2: Synthesis of 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine
[0041] In a 250mL three-neck flask, add 67g of ionic liquid [C 4 mim]PF 6, the temperature was cooled to 0°C with an ice-salt bath, and 6.05 g (0.0328 mol) of cyanuric chloride was added rapidly. After stirring evenly, start to drop 2.50g of 65% ethylamine solution (containing 1.63g of ethylamine, 0.0361mol), keep the temperature at 0°C during the dropwise addition, after the dropwise addition, at this temperature, dropwise add 30% 6.13g of potassium hydroxide solution (containing potassium hydroxide 1.84g, 0.0328mol). After the dropwise addition was completed, the stirring was continued for 30 min.
[0042] Gradually raise the temperature to 20° C., and add dropwise 2.81 g of 70% isopropylamine solution (containing 1.96 g of isopropylamine, 0.0333 mol). After the dropwise addition, at this temperature, 6.27 g of 30% potassium hydroxide solution (containing 1.88 g of potassium hydroxide,...
Embodiment 3
[0044] Example 3: Synthesis of 2-chloro-4,6-diisopropylamino-1,3,5-triazine
[0045] In a 250mL three-necked flask, add 100g of ionic liquid [C 4 mim] BF 4 , Cool the temperature to 10°C with an ice-salt bath, and quickly add 6.05 g (0.0328 mol) of cyanuric chloride. After stirring evenly, start to drop 2.88g of 70% isopropylamine solution (containing 2.02g of isopropylamine, 0.0341mol), after the dropwise addition, at this temperature, dropwise add 36.1g of 10% sodium carbonate solution (containing 2.02g of isopropylamine, 0.0341mol). 3.61 g, 0.0340 mol). After the dropwise addition was completed, the stirring was continued for 20 min.
[0046] Gradually the temperature was raised to 50°C, and 2.94g of 70% isopropylamine solution (containing 2.06g of isopropylamine, 0.0350mol) was added dropwise. After the addition, at this temperature, 37.1g of 10% sodium carbonate solution was added dropwise ( Contains sodium carbonate 3.71g, 0.0350mol). After the dropwise addition was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com