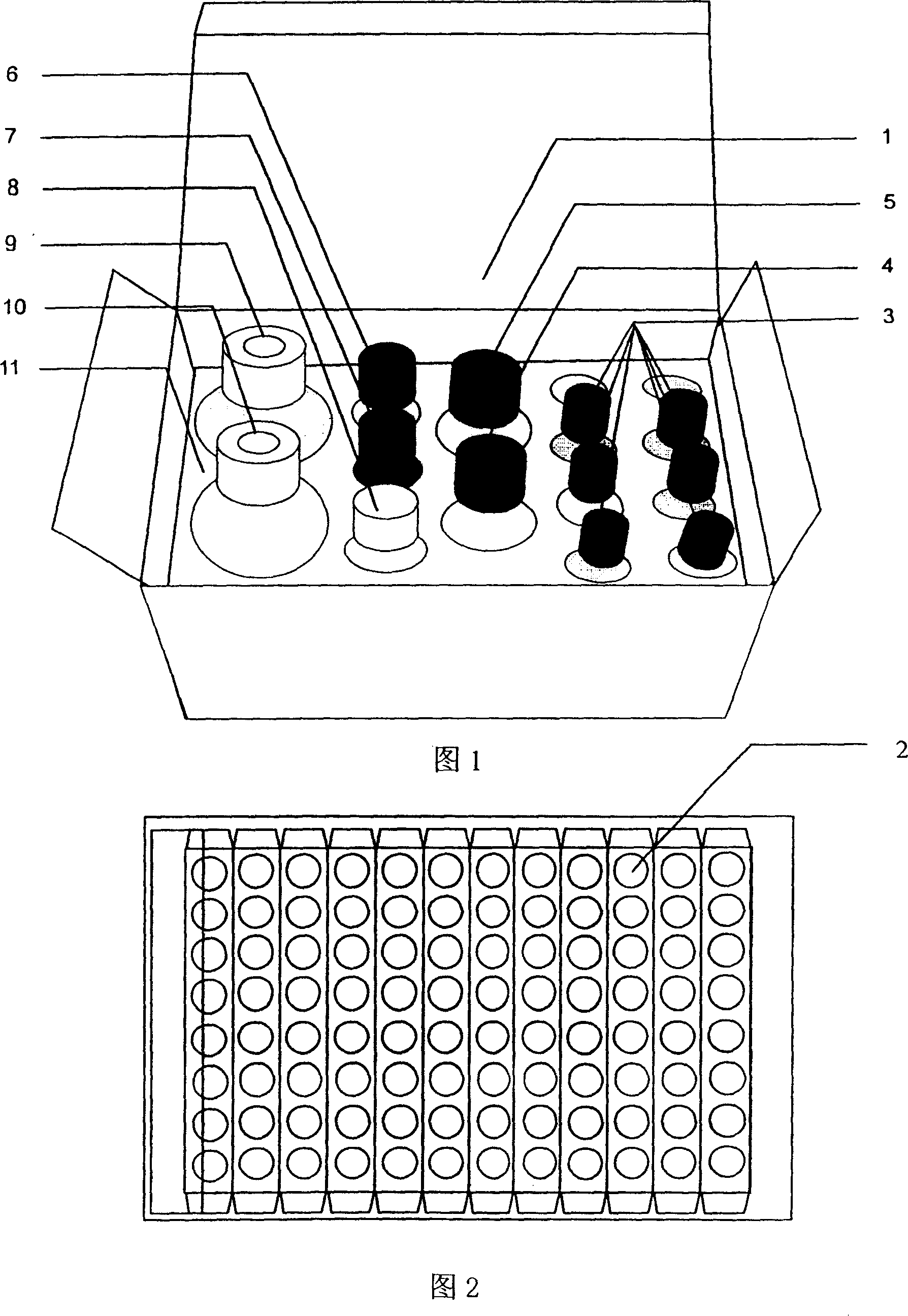
Enzyme-linked immunological kit for detecting quinoxaline medicine residue
An enzyme-linked immunosorbent reagent, quinoxaline-based technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem of inability to reflect olaquindox, cannot use olaquindox to monitor, and does not form a large-scale monitoring of quinoxaline drug immunity Kits and other problems, to achieve the effect of convenient inspection method, simple pretreatment process and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
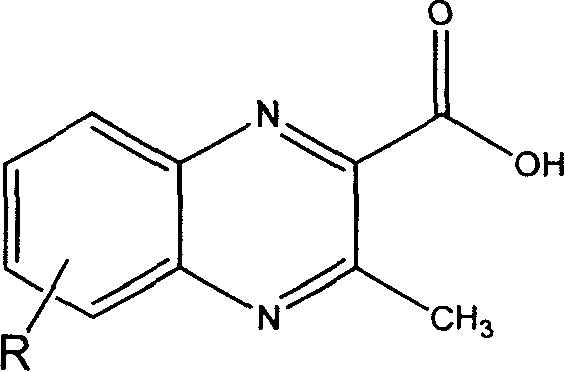
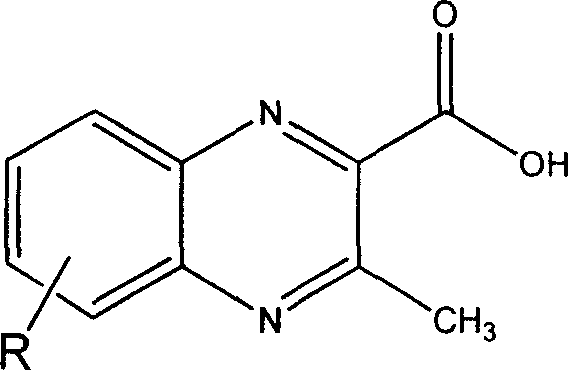
[0023] 1. The preparation of MQCA derivatives (when R is 6-CH 2 OH as an example to introduce the preparation of MQCA derivatives in detail)
[0024] a) Step 1: Dissolve 1 part of 5-hydroxymethylbenzofurazan in 2 to 6 parts of ethyl acetoacetate, and keep stirring for 6 to 8 hours in the presence of 2 to 3 parts of alkali to obtain a yellow precipitate. For 3-methyl-6-hydroxymethyl-2-ethoxycarbonylquinoxaline-1,4-dioxide. The reaction temperature is -10 to 50°C; the base used for the catalyst in the reaction can be an organic base or an inorganic base, and the organic base includes diethylamine, triethylamine, trimethylamine, n-propylamine, pyridine, picoline, piperidine, etc. Inorganic bases include sodium hydroxide, potassium hydroxide, calcium hydroxide, amine hydroxide and the like.
[0025]
[0026] b) The second step: 1 part of 3-methyl-6-hydroxymethyl-2-ethoxycarbonylquinoxaline-1,4-dioxide reacts with 3 to 6 parts of reducing agent in an appropriate amount of wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com