Solid formulation with improved solubility and stability, and method for producing said formulation
A solid preparation, soluble technology, applied in the field of solid preparations, to achieve the effects of excellent solubility, good stability, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0604] Hereinafter, the present invention is described in detail by way of examples and test examples, but the present invention is not limited to these.
[0605] will contain benzamide derivatives, 2-(2-{3-dimethylcarbamoyl-4-[(4′-trifluoromethyl-biphenyl-2-carbonyl)-amino]-phenyl} -Acetoxymethyl)-2-phenyl-diethyl malonate (hereinafter abbreviated as "MTP inhibitory compound") MTP inhibitory substance was used as a poorly soluble chemical substance in water, and was used in the following examples as active ingredient.
[0606] 1. Granulation,
[0607] (1) Dissolution of chemical substances as active ingredients
[0608] 418.5 g of acetone and 46.5 g of absolute ethanol (% by weight; 9:1) were injected into the propeller mixer, and 30.0 g of the above-mentioned MTP-inhibiting compounds of poorly water-soluble chemicals were added to the obtained acetone / absolute ethanol mixture and mix and fully dissolve.
[0609] (2) Dissolution of polymer substances
[0610] 300.0 g o...
Embodiment 2
[0622] Dissolution test
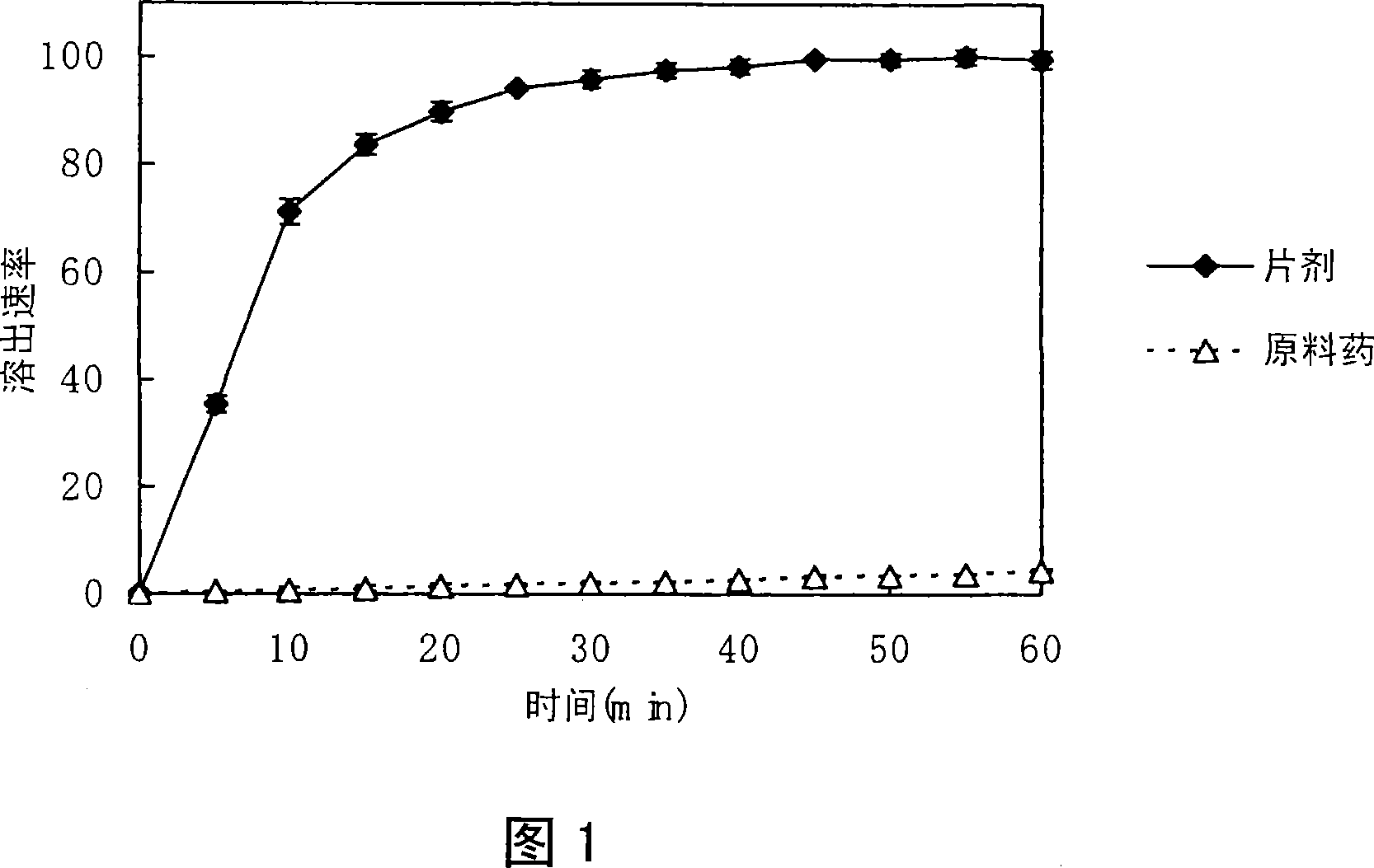
[0623] Using a 0.1 w / v% degassed sodium lauryl sulfate solution (900 mL, 37° C.), the dissolution test was performed according to the second method (paddle method, 50 rpm) of the Japanese Pharmacopoeia dissolution test method. The tablet (133.00 mg / tablet; containing 5 mg of bulk drug) prepared in Example 1 was put into the test solution as it was. For the reference control, the drug substance that constitutes the crystals is placed into the test solution as it is. Adjust the temperature to 37±0.1°C. The results are shown in Figure 1.
[0624] As shown in Fig. 1, 95.45% of the solid formulation of the present invention was dissolved within 15 minutes, while the reference compound (crystal) was not dissolved even after 60 minutes.
[0625] Considering that it is said that the food at the entrance will reach the small intestine in about 15 minutes to 30 minutes, the solid preparation of the present invention will completely dissolve when the preparat...
Embodiment 3
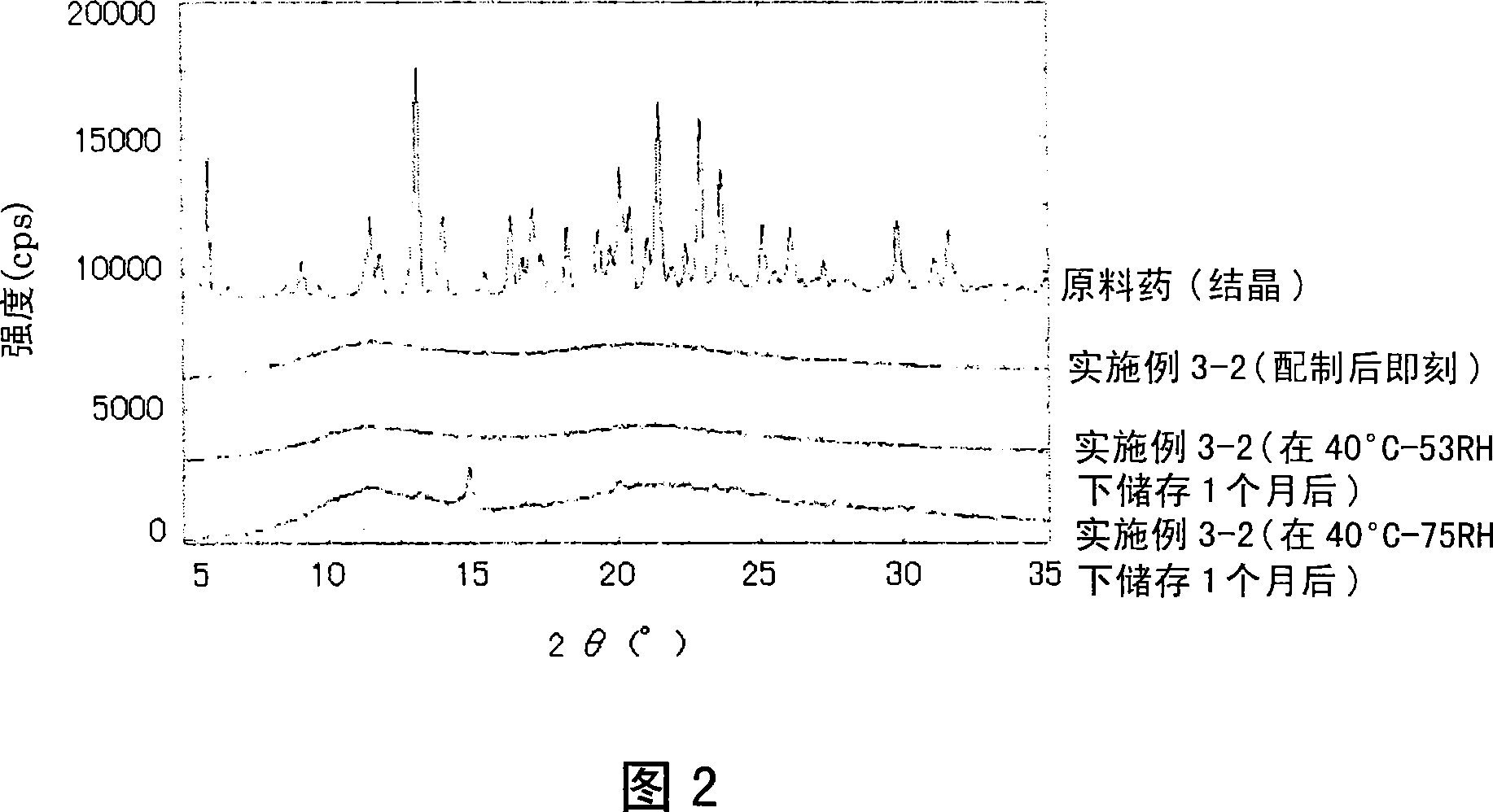
[0630] Formulations for Stability Testing and Amorphism Confirmation Testing
[0631] Test preparations were prepared in the following manner in order to confirm the stability and amorphousness of the solid preparations of the present invention.
[0632] Meanwhile, it is considered that the main cause affecting stability and crystallinity depends on the combination of the water-soluble polymer substance and the chemical substance poorly soluble in water, and a solid dispersion composed of both of these substances was used as a test preparation. The above MTP inhibitory compounds are used as poorly soluble chemicals in water.
[0633] Test formulations were prepared by adding an appropriate amount of the MTP-inhibiting compound to an appropriate amount of a suitable solvent shown below and additionally gradually adding the polymer substance to be tested, fully dissolving it, and spray-drying it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com