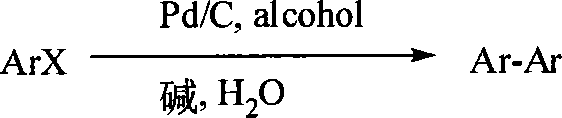Synthesis method for biphenyl compound
A synthesis method and compound technology, applied in organic chemistry methods, chemical instruments and methods, and hydrocarbon production from halogen-containing organic compounds, etc., can solve problems such as difficult operation, low yield of target products, increased costs, etc., and achieve low cost , No waste residue pollution, little impact on the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1 biphenyl
[0028] Add NaOH (8 g, 0.2 mol), H 2 O (27mL), ethylene glycol (6.2g, 0.1mol), bromobenzene (15.6g, 0.1mol), after stirring to form an emulsion, add 5% Pd / C catalyst (4.2g, 0.2mol%) at 100 After reacting at ℃ for 5 h, the reaction liquid was cooled and suction filtered. The filter cake was Pd / C catalyst, which was washed with water and could be recycled and reused; the filtrate was extracted with ethyl acetate, and the solvent was evaporated to obtain the crude product, which was recrystallized to obtain white crystal 7.1 g, yield 93%, melting point 70°C. 1 H NMR (400MHz, CDCl 3 ,) δppm: 7.60 (d, 4H, J = 7.5Hz), 7.44 (t, 4H, J = 7.2Hz), 7.35 (t, 2H, J = 7.2Hz); 13 C NMR (CDCl 3 ) δppm: 141.2, 128.7, 127.2, 127.1; MS (EI): m / z (%): 154 (100) [M + ], 153(35), 152(20), 115(3), 76(13), 51(4).
Embodiment 2
[0029] Synthesis of embodiment 2 biphenyl
[0030] In a 100mL round bottom flask, add KOH (11.2g, 0.2mol), H 2 O (27mL), ethanol (4.6g, 0.1mol), bromobenzene (15.6g, 0.1mol), after stirring to form an emulsion, add 5% Pd / C catalyst (4.2g, 0.2mol%) and react at 100°C After 5h, the reaction liquid was cooled and suction-filtered, the filter cake was Pd / C catalyst, which was washed with water, and could be recycled and reused; the filtrate was extracted with ethyl acetate, and the solvent was evaporated to obtain a crude product, which was recrystallized to obtain 7.1 g of white crystals, collected The yield is 90%, and the melting point is 70°C.
Embodiment 3
[0031] Embodiment 3 4, the synthesis of 4-dichlorobiphenyl
[0032] Add NaOH (20 g, 0.5 mol), H 2 O (45mL), ethylene glycol (32g, 0.5mol), p-bromochlorobenzene (12.8g, 0.1mol), after stirring to form an emulsion, add 5% Pd / C catalyst (10.5g, 0.5mol%) in Reaction at 100°C for 5 hours, white crystals precipitated, the reaction liquid was cooled and filtered, and the filter cake Pd / C catalyst was washed with water, which can be recycled and reused; the filtrate was extracted with ethyl acetate, and the solvent was evaporated to obtain the crude product, which was recrystallized to obtain white Crystal 7.1g, yield 89%, melting point 149°C. 1 HNMR (400MHz, CDCl 3 ) δppm: 7.48 (d, 4H, J=8.0Hz), 7.40 (d, 4H, J=8.0Hz); 13C NMR (CDCl 3 )δppm: 138.4, 133.7, 129.0, 128.2; MS (EI): m / z (%): 222 (100) [M + ], 186(15), 152(51), 111(5), 93(7), 75(9).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com